This is a preprint.
Identification of arginine-vasopressin receptor 1a (Avpr1a/AVPR1A) as a novel candidate gene for chronic visceral pain
- PMID: 38187732
- PMCID: PMC10769202
- DOI: 10.1101/2023.12.19.572390
Identification of arginine-vasopressin receptor 1a (Avpr1a/AVPR1A) as a novel candidate gene for chronic visceral pain
Update in
-
Identification of Arginine-Vasopressin Receptor 1a (Avpr1a/Avpr1a) as a Novel Candidate Gene for Chronic Visceral Pain Sheds Light on the Potential Role of Enteric Neurons in the Development of Visceral Hypersensitivity.J Pain. 2024 Sep;25(9):104572. doi: 10.1016/j.jpain.2024.104572. Epub 2024 May 18. J Pain. 2024. PMID: 38768798 Free PMC article.
Abstract
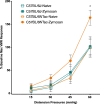
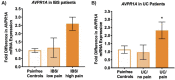
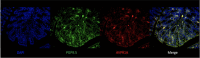
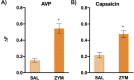
Chronic abdominal pain in the absence of ongoing disease is the hallmark of disorders of gut-brain interaction (DGBIs), including irritable bowel syndrome (IBS). While the etiology of DGBIs remains poorly understood, there is evidence that both genetic and environmental factors play a role. In this study, we report the identification and validation of Avpr1a as a novel candidate gene for visceral hypersensitivity (VH), a primary peripheral mechanism underlying abdominal pain in DGBI/IBS. Comparing two C57BL/6 (BL/6) substrains (C57BL/6NTac and C57BL/6J) revealed differential susceptibility to the development of chronic VH following intrarectal zymosan (ZYM) instillation, a validated preclinical model for post-inflammatory IBS. Using whole genome sequencing, we identified a SNP differentiating the two strains in the 5' intergenic region upstream of Avpr1a, encoding the protein arginine-vasopressin receptor 1A (AVPR1A). We used behavioral, histological, and molecular approaches to identify distal colon-specific gene expression differences and neuronal hyperresponsiveness covarying with Avpr1a genotype and VH susceptibility. While the two BL/6 substrains did not differ across other gastrointestinal (GI) phenotypes (e.g., GI motility), VH-susceptible BL/6NTac mice had higher colonic Avpr1a mRNA and protein expression. Moreover, neurons of the enteric nervous system were hyperresponsive to the AVPR1A agonist AVP, suggesting a role for enteric neurons in the pathology underlying VH. These results parallel our findings that patients' colonic Avpr1a mRNA expression was higher in patients with higher pain ratings. Taken together, these findings implicate differential regulation of Avpr1a as a novel mechanism of VH-susceptibility as well as a potential therapeutic target specific to VH.
Keywords: disorders of gut-brain interaction; enteric nervous system; genetics; irritable bowel syndrome; visceral hypersensitivity.
Conflict of interest statement
Conflict of Interest The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
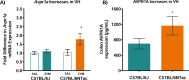

Similar articles
-
Identification of Arginine-Vasopressin Receptor 1a (Avpr1a/Avpr1a) as a Novel Candidate Gene for Chronic Visceral Pain Sheds Light on the Potential Role of Enteric Neurons in the Development of Visceral Hypersensitivity.J Pain. 2024 Sep;25(9):104572. doi: 10.1016/j.jpain.2024.104572. Epub 2024 May 18. J Pain. 2024. PMID: 38768798 Free PMC article.
-
Subtle sex differences in vasopressin mRNA expression in the embryonic mouse brain.J Neuroendocrinol. 2020 Feb;32(2):e12835. doi: 10.1111/jne.12835. Epub 2020 Feb 12. J Neuroendocrinol. 2020. PMID: 31961993 Free PMC article.
-
Sensitivity testing in irritable bowel syndrome with rectal capsaicin stimulations: role of TRPV1 upregulation and sensitization in visceral hypersensitivity?Am J Gastroenterol. 2014 Jan;109(1):99-109. doi: 10.1038/ajg.2013.371. Epub 2013 Nov 5. Am J Gastroenterol. 2014. PMID: 24189713
-
The Role of Visceral Hypersensitivity in Irritable Bowel Syndrome: Pharmacological Targets and Novel Treatments.J Neurogastroenterol Motil. 2016 Oct 30;22(4):558-574. doi: 10.5056/jnm16001. J Neurogastroenterol Motil. 2016. PMID: 27431236 Free PMC article. Review.
-
Molecular genetic studies of the arginine vasopressin 1a receptor (AVPR1a) and the oxytocin receptor (OXTR) in human behaviour: from autism to altruism with some notes in between.Prog Brain Res. 2008;170:435-49. doi: 10.1016/S0079-6123(08)00434-2. Prog Brain Res. 2008. PMID: 18655900 Review.
References
-
- Mayer E.A. and Gebhart G.F., Basic and clinical aspects of visceral hyperalgesia. Gastroenterology, 1994. 107(1): p. 271–93. - PubMed
-
- Camilleri M., Saslow S.B., and Bharucha A.E., Gastrointestinal sensation. Mechanisms and relation to functional gastrointestinal disorders. Gastroenterol Clin North Am, 1996. 25(1): p. 247–58. - PubMed
-
- Barbara G., et al., New pathophysiological mechanisms in irritable bowel syndrome. Aliment Pharmacol Ther, 2004. 20 Suppl 2: p. 1–9. - PubMed
-
- Sayuk G.S., et al., Opioid medication use in patients with gastrointestinal diagnoses vs unexplained gastrointestinal symptoms in the US Veterans Health Administration. Aliment Pharmacol Ther, 2018. 47(6): p. 784–791. - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous
