This is a preprint.
Structure of the human systemic RNAi defective transmembrane protein 1 (hSIDT1) reveals the conformational flexibility of its lipid binding domain
- PMID: 38187772
- PMCID: PMC10769365
- DOI: 10.1101/2023.12.21.572875
Structure of the human systemic RNAi defective transmembrane protein 1 (hSIDT1) reveals the conformational flexibility of its lipid binding domain
Update in
-
Structure of the human systemic RNAi defective transmembrane protein 1 (hSIDT1) reveals the conformational flexibility of its lipid binding domain.Life Sci Alliance. 2024 Jun 26;7(9):e202402624. doi: 10.26508/lsa.202402624. Print 2024 Sep. Life Sci Alliance. 2024. PMID: 38925866 Free PMC article.
Abstract
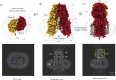
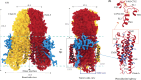
In C. elegans, inter-cellular transport of the small non-coding RNA causing systemic RNA interference (RNAi) is mediated by the transmembrane protein SID1, encoded by the sid1 gene in the systemic RNA interference-defective (sid) loci. SID1 shares structural and sequence similarity with cholesterol uptake protein 1 (CHUP1) and is classified as a member of the cholesterol uptake family (ChUP). Although systemic RNAi is not an evolutionarily conserved process, the sid gene products are found across the animal kingdom, suggesting the existence of other novel gene regulatory mechanisms mediated by small non-coding RNAs. Human homologs of sid gene products - hSIDT1 and hSIDT2 - mediate contact-dependent lipophilic small non-coding dsRNA transport. Here, we report the structure of recombinant human SIDT1. We find that the extra-cytosolic domain (ECD) of hSIDT1 adopts a double jelly roll fold, and the transmembrane domain (TMD) exists as two modules - a flexible lipid binding domain (LBD) and a rigid TMD core. Our structural analyses provide insights into the inherent conformational dynamics within the lipid binding domain in cholesterol uptake (ChUP) family members.
Conflict of interest statement
Conflict of interest The authors declare no conflict of interest.
Figures




References
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
