Product analog binding identifies the copper active site of particulate methane monooxygenase
- PMID: 38187819
- PMCID: PMC10766429
- DOI: 10.1038/s41929-023-01051-x
Product analog binding identifies the copper active site of particulate methane monooxygenase
Abstract
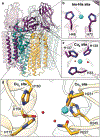
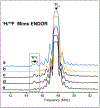
Nature's primary methane-oxidizing enzyme, the membrane-bound particulate methane monooxygenase (pMMO), catalyzes the oxidation of methane to methanol. pMMO activity requires copper, and decades of structural and spectroscopic studies have sought to identify the active site among three candidates: the CuB, CuC, and CuD sites. Challenges associated with the isolation of active pMMO have hindered progress toward locating its catalytic center. However, reconstituting pMMO into native lipid nanodiscs stabilizes its structure and recovers its activity. Here, these active samples were incubated with 2,2,2,-trifluoroethanol (TFE), a product analog that serves as a readily visualized active-site probe. Interactions of TFE with the CuD site were observed by both pulsed ENDOR spectroscopy and cryoEM, implicating CuD and the surrounding hydrophobic pocket as the likely site of methane oxidation. Use of these orthogonal techniques on parallel samples is a powerful approach that can circumvent difficulties in interpreting metalloenzyme cryoEM maps.
Figures



References
-
- Arndtsen BA, Bergman RG, Mobley TA & Peterson TH Selective intermolecular carbon-hydrogen bond activation by synthetic metal complexes in homogeneous solution. Acc. Chem. Res 28, 154–162 (1995).
-
- Blanksby SJ & Ellison GB Bond dissociation energies of organic molecules. Acc. Chem. Res 36, 255–263 (2003). - PubMed
-
- Tang P, Zhu QJ, Wu ZX & Ma D Methane activation: the past and future. Energy Environ. Sci 7, 2580–2591 (2014).
-
- Haynes CA & Gonzalez R Rethinking biological activation of methane and conversion to liquid fuels. Nat. Chem. Biol 10, 331–339 (2014). - PubMed
-
- Ravi M, Ranocchiari M & van Bokhoven JA The direct catalytic oxidation of methane to methanol-a critical assessment. Angew. Chem. Int. Ed. Engl 56, 16464–16483 (2017). - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
