Metabolites in the regulatory risk assessment of pesticides in the EU
- PMID: 38188093
- PMCID: PMC10770266
- DOI: 10.3389/ftox.2023.1304885
Metabolites in the regulatory risk assessment of pesticides in the EU
Abstract
A large majority of chemicals is converted into metabolites through xenobiotic-metabolising enzymes. Metabolites may present a spectrum of characteristics varying from similar to vastly different compared with the parent compound in terms of both toxicokinetics and toxicodynamics. In the pesticide arena, the role of metabolism and metabolites is increasingly recognised as a significant factor particularly for the design and interpretation of mammalian toxicological studies and in the toxicity assessment of pesticide/metabolite-associated issues for hazard characterization and risk assessment purposes, including the role of metabolites as parts in various residues in ecotoxicological adversities. This is of particular relevance to pesticide metabolites that are unique to humans in comparison with metabolites found in in vitro or in vivo animal studies, but also to disproportionate metabolites (quantitative differences) between humans and mammalian species. Presence of unique or disproportionate metabolites may underlie potential toxicological concerns. This review aims to present the current state-of-the-art of comparative metabolism and metabolites in pesticide research for hazard and risk assessment, including One Health perspectives, and future research needs based on the experiences gained at the European Food Safety Authority.
Keywords: analytical methods; disproportionate human metabolite; in vitro/in silico testing; pesticide metabolite; risk assessment; unique human metabolite; xenobiotic metabolism.
Copyright © 2023 Pelkonen, Abass, Parra Morte, Panzarea, Testai, Rudaz, Louisse, Gundert-Remy, Wolterink, Jean-Lou CM, Coecke and Bernasconi.
Conflict of interest statement
JoL, JeL, MP and JP were employed by the European Food Safety Authority (EFSA). SC and CB were employed by the European Commission Joint Research Centre (JRC). The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
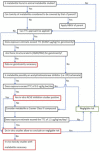
Figures



References
Publication types
LinkOut - more resources
Full Text Sources

