Prevalence of chronic HCV infection in EU/EEA countries in 2019 using multiparameter evidence synthesis
- PMID: 38188273
- PMCID: PMC10769889
- DOI: 10.1016/j.lanepe.2023.100792
Prevalence of chronic HCV infection in EU/EEA countries in 2019 using multiparameter evidence synthesis
Abstract
Background: Epidemiological data are crucial to monitoring progress towards the 2030 Hepatitis C Virus (HCV) elimination targets. Our aim was to estimate the prevalence of chronic HCV infection (cHCV) in the European Union (EU)/European Economic Area (EEA) countries in 2019.
Methods: Multi-parameter evidence synthesis (MPES) was used to produce national estimates of cHCV defined as: π = πrecρrec + πexρex + πnonρnon; πrec, πex, and πnon represent cHCV prevalence among recent people who inject drugs (PWID), ex-PWID, and non-PWID, respectively, while ρrec, ρex, and ρnon represent the proportions of these groups in the population. Information sources included the European Centre for Disease Prevention and Control (ECDC) national operational contact points (NCPs) and prevalence database, the European Monitoring Centre for Drugs and Drug Addiction databases, and the published literature.
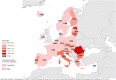
Findings: The cHCV prevalence in 29 of 30 EU/EEA countries in 2019 was 0.50% [95% Credible Interval (CrI): 0.46%, 0.55%]. The highest cHCV prevalence was observed in the eastern EU/EEA (0.88%; 95% CrI: 0.81%, 0.94%). At least 35.76% (95% CrI: 33.07%, 38.60%) of the overall cHCV prevalence in EU/EEA countries was associated with injecting drugs.
Interpretation: Using MPES and collaborating with ECDC NCPs, we estimated the prevalence of cHCV in the EU/EEA to be low. Some areas experience higher cHCV prevalence while a third of prevalent cHCV infections was attributed to PWID. Further efforts are needed to scale up prevention measures and the diagnosis and treatment of infected individuals, especially in the east of the EU/EEA and among PWID.
Funding: ECDC.
Keywords: Chronic hepatitis; Elimination; Europe; HCV; Hepatitis C; Prevalence.
© 2023 The Author(s).
Conflict of interest statement
IG: He is currently an employee of MSD Greece. He joined MSD after his post-doctoral work at the University of Cyprus. TV: He has received grants from Gilead Sciences and Bristol Myers Squibb; he has served as a consultant for Janssen Pharmaceuticals, Gilead Sciences, AbbVie, Bristol Myers Squibb; and he has served as a sponsored lecturer for Gilead Sciences and Abbvie. PBC: He has received unrestricted research grants for other studies from Abbvie, Gilead, and MSD. MG: He has received consultancy and speaker’s fees from Gilead Sciences. LAK: She has received personal lecturer fee from Abbvie and Gilead Sciences and an institutional grant from Gilead Italy Fellowship 2022. LJ: She has received honorarium for lectures from AbbVie and MSD; offered consultancy to AbbVie, MSD, Tamro; and received conference attending fee from AbbVie, MSD, Pfizer, Swixx Biopharma. CSD: She has received educational and research grants for other studies from Abbvie and Gilead Sciences. MV: He participated in advisory boards (ViiV, Gilead, and MSD–fees paid to his institution); he has received independent research grants from ViiV and Gilead (paid to his institution). CV: She has received honorarium for lectures and consultancy from AbbVie, Gilead, MSD, and ViiV Healthcare. AD: She has received a grant for another study and speaker fee at a conference about HIV from Gilead Sciences. AB: He has received speaker's fees from Gilead Sciences. GN: He has received an ASKLEPIOS grant (HIV-related competitive grant) from Gilead Sciences (Greece). All other authors declare no conflict of interest.
Figures

References
-
- Polaris Observatory Collaborators Global change in hepatitis C virus prevalence and cascade of care between 2015 and 2020: a modelling study. Lancet Gastroenterol Hepatol. 2022;7:396–415. - PubMed
-
- European Association for the Study of the Liver EASL recommendations on treatment of hepatitis C: final update of the series. J Hepatol. 2020;73:1170–1218. - PubMed
-
- World Health Organization (WHO) WHO; Geneva: 2016. Global health sector strategy on viral hepatitis, 2016–2021.https://www.who.int/publications/i/item/WHO-HIV-2016.06
-
- Lombardi A., Mondelli M.U. ESCMID study group for viral hepatitis (ESGVH). Hepatitis C: is eradication possible? Liver Int. 2019;39:416–426. - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

