Metabolic pseudoprogression in a patient with metastatic KIT exon 11 GIST after 1 month of first-line imatinib: a case report
- PMID: 38188286
- PMCID: PMC10769864
- DOI: 10.3389/fonc.2023.1310452
Metabolic pseudoprogression in a patient with metastatic KIT exon 11 GIST after 1 month of first-line imatinib: a case report
Abstract
Background: Positron emission tomography (PET) with 18-fluorodeoxyglucose (18FDG) has proven to be highly sensitive in the early assessment of tumor response in gastrointestinal stromal tumors (GIST), especially in cases where there is doubt or when the early prediction of the response could be clinically useful for patient management. As widely known, kinase mutations have an undoubtful predictive value for sensitivity to imatinib, and the inclusion of KIT and PDGFRa mutational analysis in the diagnostic workup of all GIST is now considered standard practice.
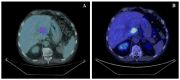
Case presentation: Herein, we described in detail a case of an exon 11 KIT mutated-metastatic GIST patient, who presented an unexpected metabolic progression at the early 18FDG-PET evaluation after 1 month of first-line imatinib, unconfirmed at the liver biopsy performed near after, which has conversely shown a complete pathological response.
Conclusions: This report aims to highlight the existence of this metabolic pseudoprogression in GIST at the beginning of imatinib therapy in order to avoid early treatment discontinuation. Therefore, an early metabolic progression during a molecular targeted therapy always deserves to be evaluated in the context of the disease molecular profiling, and in case of a discordant finding between functional imaging and molecular background, a short-term longitudinal control should be suggested.
Keywords: FDG-PET; GIST; functional imaging; gastrointestinal stromal tumors; imatinib.
Copyright © 2023 Tassinari, Conci, Battisti, Porta, Di Scioscio, Pirini, de Biase, Nigro, Iezza, Castagnetti, Lovato, Fanti, Pantaleo and Nannini.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures




References
Publication types
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

