Aedes aegypti saliva modulates inflammasome activation and facilitates flavivirus infection in vitro
- PMID: 38188518
- PMCID: PMC10770497
- DOI: 10.1016/j.isci.2023.108620
Aedes aegypti saliva modulates inflammasome activation and facilitates flavivirus infection in vitro
Abstract
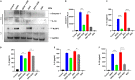
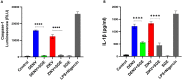
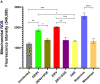
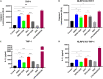
Mosquito borne flaviviruses such as dengue and Zika represent a major public health problem due to globalization and propagation of susceptible vectors worldwide. Vertebrate host responses to dengue and Zika infections include the processing and release of pro-inflammatory cytokines through the activation of inflammasomes, resulting in disease severity and fatality. Mosquito saliva can facilitate pathogen infection by downregulating the host's immune response. However, the role of mosquito saliva in modulating host innate immune responses remains largely unknown. Here, we show that mosquito salivary gland extract (SGE) inhibits dengue and Zika virus-induced inflammasome activation by reducing NLRP3 expression, Caspase-1 activation, and 1L-1β secretion in cultured human and mice macrophages. As a result, we observe that SGE inhibits virus detection in the early phase of infection. This study provides important insights into how mosquito saliva modulates host innate immunity during viral infection.
Keywords: Biological sciences; Natural sciences; Virology.
Conflict of interest statement
The authors declare no competing interests.
Figures

Similar articles
-
Aedes aegypti NeSt1 Protein Enhances Zika Virus Pathogenesis by Activating Neutrophils.J Virol. 2019 Jun 14;93(13):e00395-19. doi: 10.1128/JVI.00395-19. Print 2019 Jul 1. J Virol. 2019. PMID: 30971475 Free PMC article.
-
Co-infection of dengue and Zika viruses mutually enhances viral replication in the mosquito Aedes aegypti.Parasit Vectors. 2023 May 11;16(1):160. doi: 10.1186/s13071-023-05778-1. Parasit Vectors. 2023. PMID: 37165438 Free PMC article.
-
Cell-Fusing Agent Virus Reduces Arbovirus Dissemination in Aedes aegypti Mosquitoes In Vivo.J Virol. 2019 Aug 28;93(18):e00705-19. doi: 10.1128/JVI.00705-19. Print 2019 Sep 15. J Virol. 2019. PMID: 31243123 Free PMC article.
-
Flaviviruses: Innate Immunity, Inflammasome Activation, Inflammatory Cell Death, and Cytokines.Front Immunol. 2022 Jan 28;13:829433. doi: 10.3389/fimmu.2022.829433. eCollection 2022. Front Immunol. 2022. PMID: 35154151 Free PMC article. Review.
-
Mosquito Saliva: The Hope for a Universal Arbovirus Vaccine?J Infect Dis. 2018 Jun 5;218(1):7-15. doi: 10.1093/infdis/jiy179. J Infect Dis. 2018. PMID: 29617849 Free PMC article. Review.
Cited by
-
Unraveling the Molecular Mechanisms of Mosquito Salivary Proteins: New Frontiers in Disease Transmission and Control.Biomolecules. 2025 Jan 8;15(1):82. doi: 10.3390/biom15010082. Biomolecules. 2025. PMID: 39858476 Free PMC article. Review.
-
Structural and functional significance of Aedes aegypti AgBR1 flavivirus immunomodulator.J Virol. 2025 May 20;99(5):e0187824. doi: 10.1128/jvi.01878-24. Epub 2025 Apr 24. J Virol. 2025. PMID: 40272158 Free PMC article.
-
Transient Expression of Zika NS2B Protein Antigen in Nicotiana benthamiana and Use for Arboviruses Diagnosis.ACS Omega. 2025 Jan 8;10(2):2184-2196. doi: 10.1021/acsomega.4c08998. eCollection 2025 Jan 21. ACS Omega. 2025. PMID: 39866622 Free PMC article.
References
-
- WHO Vector-borne diseases. 2020. https://www.who.int/news-room/fact-sheets/detail/vector-borne-diseases
-
- Lupton K. Zika virus disease: a public health emergency of international concern. Br. J. Nurs. 2016;25:198–200. - PubMed
LinkOut - more resources
Full Text Sources
Research Materials

