Randomized trial of ketamine masked by surgical anesthesia in patients with depression
- PMID: 38188539
- PMCID: PMC10769130
- DOI: 10.1038/s44220-023-00140-x
Randomized trial of ketamine masked by surgical anesthesia in patients with depression
Abstract
Ketamine may have antidepressant properties, but its acute psychoactive effects complicate successful masking in placebo-controlled trials. We present a single-center, parallel-arm, triple-masked, randomized, placebo-controlled trial assessing the antidepressant efficacy of intravenous ketamine masked by surgical anesthesia (ClinicalTrials.gov, NCT03861988). Forty adult patients with major depressive disorder who were scheduled for routine surgery were randomized to a single infusion of ketamine (0.5 mg/kg) or placebo (saline) during usual anesthesia. All participants, investigators, and direct patient care staff were masked to treatment allocation. The primary outcome was depression severity measured by the Montgomery-Åsberg Depression Rating Scale (MADRS) at 1, 2, and 3 days post-infusion. After all follow-up visits, participants were asked to guess which intervention they received. A mixed-effects model showed no evidence of effect of treatment assignment on the primary outcome (-5.82, 95% CI -13.3 to 1.64, p=0.13). 36.8% of participants guessed their treatment assignment correctly; both groups allocated their guesses in similar proportions. In conclusion, a single dose of intravenous ketamine delivered during surgical anesthesia had no greater effect than placebo in acutely reducing the severity of depressive symptoms in adults with major depressive disorder. This trial successfully masked treatment allocation in moderate-to-severely depressed patients using surgical anesthesia. Although this masking strategy is impractical for most placebo-controlled trials, future studies of novel antidepressants with acute psychoactive effects should make efforts to fully mask treatment assignment in order to minimize subject-expectancy bias.
Conflict of interest statement
Competing Interests Statement B.D.H. is on the scientific advisory boards of Osmind and Journey Clinical and is a consultant for Clairvoyant Therapeutics and Vine Ventures. Dr. Schatzberg has served as a consultant to Alto Neuroscience, ANeurotech, Compass, Magnus, NeuraWell, Parexal, Sage and Signant. He holds equity in Alto Neuroscience, Corcept, Delpor, Madrigal, Magnus, Seattle Genetics, Titan and Xhale. These interests had no role in the present trial. The other authors declare no competing interests.
Figures
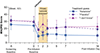

Update of
-
Randomized Trial of Ketamine Masked by Surgical Anesthesia in Depressed Patients.medRxiv [Preprint]. 2023 Jun 15:2023.04.28.23289210. doi: 10.1101/2023.04.28.23289210. medRxiv. 2023. Update in: Nat Ment Health. 2023 Nov;1(11):876-886. doi: 10.1038/s44220-023-00140-x. PMID: 37205558 Free PMC article. Updated. Preprint.
References
-
- Sleigh J, Harvey M, Voss L & Denny B Ketamine – More mechanisms of action than just NMDA blockade. Trends in Anaesthesia and Critical Care 4, 76–81 (2014).
-
- Berman RM et al. Antidepressant effects of ketamine in depressed patients. Biological Psychiatry 47, 351–354 (2000). - PubMed
-
- Zarate CA et al. A Randomized Trial of an N-methyl-D-aspartate Antagonist in Treatment-Resistant Major Depression. Arch Gen Psychiatry 63, 856–864 (2006). - PubMed
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
