Intranasal Immunization With Nanoparticles Containing an Orientia tsutsugamushi Protein Vaccine Candidate and a Polysorbitol Transporter Adjuvant Enhances Both Humoral and Cellular Immune Responses
- PMID: 38188601
- PMCID: PMC10767547
- DOI: 10.4110/in.2023.23.e47
Intranasal Immunization With Nanoparticles Containing an Orientia tsutsugamushi Protein Vaccine Candidate and a Polysorbitol Transporter Adjuvant Enhances Both Humoral and Cellular Immune Responses
Abstract
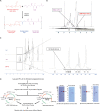
Scrub typhus, a mite-borne infectious disease, is caused by Orientia tsutsugamushi. Despite many attempts to develop a protective strategy, an effective preventive vaccine has not been developed. The identification of appropriate Ags that cover diverse antigenic strains and provide long-lasting immunity is a fundamental challenge in the development of a scrub typhus vaccine. We investigated whether this limitation could be overcome by harnessing the nanoparticle-forming polysorbitol transporter (PST) for an O. tsutsugamushi vaccine strategy. Two target proteins, 56-kDa type-specific Ag (TSA56) and surface cell Ag A (ScaA) were used as vaccine candidates. PST formed stable nano-size complexes with TSA56 (TSA56-PST) and ScaA (ScaA-PST); neither exhibited cytotoxicity. The formation of Ag-specific IgG2a, IgG2b, and IgA in mice was enhanced by intranasal vaccination with TSA56-PST or ScaA-PST. The vaccines containing PST induced Ag-specific proliferation of CD8+ and CD4+ T cells. Furthermore, the vaccines containing PST improved the mouse survival against O. tsutsugamushi infection. Collectively, the present study indicated that PST could enhance both Ag-specific humoral immunity and T cell response, which are essential to effectively confer protective immunity against O. tsutsugamushi infection. These findings suggest that PST has potential for use in an intranasal vaccination strategy.
Keywords: Adaptive immunity; Intranasal administration; Nano-vaccine; Orientia tsutsugamushi.
Copyright © 2023. The Korean Association of Immunologists.
Conflict of interest statement
Conflict of Interest: The authors declare no potential conflicts of interest.
Figures


Similar articles
-
Protective immunity of 56-kDa type-specific antigen of Orientia tsutsugamushi causing scrub typhus.J Microbiol Biotechnol. 2014 Dec 28;24(12):1728-35. doi: 10.4014/jmb.1407.07048. J Microbiol Biotechnol. 2014. PMID: 25112312
-
Immunization with an autotransporter protein of Orientia tsutsugamushi provides protective immunity against scrub typhus.PLoS Negl Trop Dis. 2015 Mar 13;9(3):e0003585. doi: 10.1371/journal.pntd.0003585. eCollection 2015 Mar. PLoS Negl Trop Dis. 2015. PMID: 25768004 Free PMC article.
-
Immunization with a recombinant antigen composed of conserved blocks from TSA56 provides broad genotype protection against scrub typhus.Emerg Microbes Infect. 2019;8(1):946-958. doi: 10.1080/22221751.2019.1632676. Emerg Microbes Infect. 2019. PMID: 31237478 Free PMC article.
-
Approaches to vaccines against Orientia tsutsugamushi.Front Cell Infect Microbiol. 2013 Jan 4;2:170. doi: 10.3389/fcimb.2012.00170. eCollection 2012. Front Cell Infect Microbiol. 2013. PMID: 23316486 Free PMC article. Review.
-
A scrub typhus vaccine presents a challenging unmet need.NPJ Vaccines. 2023 Feb 9;8(1):11. doi: 10.1038/s41541-023-00605-1. NPJ Vaccines. 2023. PMID: 36759505 Free PMC article. Review.
Cited by
-
A CRISPR-Cas12a-based universal rapid scrub typhus diagnostic method targeting 16S rRNA of Orientia tsutsugamushi.PLoS Negl Trop Dis. 2025 Jan 22;19(1):e0012826. doi: 10.1371/journal.pntd.0012826. eCollection 2025 Jan. PLoS Negl Trop Dis. 2025. PMID: 39841710 Free PMC article.
-
Exploring OmpA of Orientia tsutsugamushi to design novel multi-epitope vaccine against scrub typhus: an immunoinformatics approach.Mol Divers. 2025 Jul 9. doi: 10.1007/s11030-025-11236-0. Online ahead of print. Mol Divers. 2025. PMID: 40632361
-
RSV Vaccine with Nanoparticle-Based Poly-Sorbitol Transporter (PST) Adjuvant Improves Respiratory Protection Against RSV Through Inducing Both Systemic and Mucosal Humoral Immunity.Vaccines (Basel). 2024 Nov 29;12(12):1354. doi: 10.3390/vaccines12121354. Vaccines (Basel). 2024. PMID: 39772016 Free PMC article.
-
Concatenated ScaA and TSA56 Surface Antigen Sequences Reflect Genome-Scale Phylogeny of Orientia tsutsugamushi: An Analysis Including Two Genomes from Taiwan.Pathogens. 2024 Apr 3;13(4):299. doi: 10.3390/pathogens13040299. Pathogens. 2024. PMID: 38668254 Free PMC article.
References
-
- Banerjee A, Kulkarni S. Orientia tsutsugamushi: the dangerous yet neglected foe from the East. Int J Med Microbiol. 2021;311:151467. - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

