Effect of Exosomes From Bone Marrow-Derived Mesenchymal Stromal Cells and Adipose-Derived Stromal Cells on Bone-Tendon Healing in a Murine Rotator Cuff Injury Model
- PMID: 38188618
- PMCID: PMC10768594
- DOI: 10.1177/23259671231210304
Effect of Exosomes From Bone Marrow-Derived Mesenchymal Stromal Cells and Adipose-Derived Stromal Cells on Bone-Tendon Healing in a Murine Rotator Cuff Injury Model
Abstract
Background: Bone-tendon injury is characterized by poor self-healing. It is established that exosomes are favorable for tissue repair and regeneration. However, their effect on bone-tendon healing has not yet been determined.
Purpose: To compare the effectiveness of exosomes derived from adipose-derived mesenchymal stromal cells (ADSC-Exos) and bone marrow-derived mesenchymal stromal cells (BMSC-Exos) on bone-tendon interface healing in murine rotator cuff injury model and explore the underlying mechanisms thereof.
Study design: Controlled laboratory study.
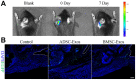
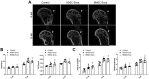
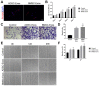
Methods: A total of 63 male C57BL6 mice with rotator cuff injuries underwent surgery and were randomly assigned to a control group treated without exosomes (n = 21), an ADSC-Exos group (n = 21), or a BMSC-Exos group (n = 21). The mice were sacrificed 4 or 8 weeks after surgery, and tissues were collected for histologic examination and radiographic and biomechanical testing. For exosome tracing in vivo, mice were sacrificed 7 days after surgery. A series of functional assays (radiographic evaluation, proliferation assay, Alizarin Red staining, alkaline phosphatase staining and activity, Alcian blue staining, quantitative polymerase chain reaction analyses, and glycosaminoglycans quantification) were conducted to evaluate the effect of exosomes on the cellular behaviors of the BMSCs in vitro. A statistical analysis of multiple-group comparisons was performed by 1-way analysis of variance, followed by the Bonferroni post hoc test to assess the differences between the 2 groups.
Results: The ADSCs and BMSCs were positive for surface markers CD29 and CD90 and negative for surface markers CD34 and CD45 and could differentiate into osteoblasts, chondrocytes, and adipocytes. Exosomes showed a cup- or sphere-shaped morphology and were positive for CD63 and TGS101. Local injection of ADSC-Exos and BMSC-Exos could recruit BMSCs and promote osteogenesis, chondrogenesis, and bone-tendon healing. In vitro, ADSC-Exos and BMSC-Exos could significantly promote the proliferation, migration, osteogenic differentiation, and chondrogenic differentiation ability of BMSCs. In vivo, ADSC-Exos and BMSC-Exos significantly accelerated bone-tendon injury healing, with no significant statistical difference between them.
Conclusion: ADSC-Exos and BMSC-Exos exhibited similar therapeutic effects on bone-tendon healing in our murine animal model.
Clinical relevance: ADSC-Exos and BMSC-Exos may be used to develop a new cell-free therapy method for promoting rotator cuff injury repair.
Keywords: adipose-derived mesenchymal stromal cell; bone marrow–derived mesenchymal stromal cell; bone-tendon healing; exosomes; rotator cuff.
© The Author(s) 2024.
Conflict of interest statement
One or more of the authors has declared the following potential conflict of interest or source of funding: Research support was received from the National Key Clinical Specialty Construction Project at Pediatric Surgery of Hunan Children's Hospital (XWYF [2022] No. 2), the Hunan Province Science and Technology Innovation Plan Project (2021SK50516), and the 440 Young Talents of 1233 program of Hunan Children Hospital. AOSSM checks author disclosures against the Open Payments Database (OPD). AOSSM has not conducted an independent investigation on the OPD and disclaims any liability or responsibility relating thereto. Ethical approval for this study was obtained from the Animal Care and Use Committee of Hunan Children's Hospital (ref No: HCHDWLL-2022-16).
Figures



References
-
- Basu J, Ludlow JW. Exosomes for repair, regeneration and rejuvenation. Expert Opin Biol Ther. 2016;16:489-506. - PubMed
-
- Cavallo C, Cuomo C, Fantini S, et al. Comparison of alternative mesenchymal stem cell sources for cell banking and musculoskeletal advanced therapies. J Cell Biochem. 2011;112:1418-1430. - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

