Role of intestinal flora in the development of nonalcoholic fatty liver disease in children
- PMID: 38189294
- PMCID: PMC10846053
- DOI: 10.1128/spectrum.01006-23
Role of intestinal flora in the development of nonalcoholic fatty liver disease in children
Abstract
In China, 45% of adolescents with obesity develop fatty liver disease, a condition that increases the long-term risk of developing cirrhosis and liver cancer. Although the factors triggering nonalcoholic fatty liver disease (NAFLD) vary in children, the composition of intestinal microflora has been found to play an increasingly important role. However, evidence is limited on the prevalence of nonalcoholic fatty liver (NAFL) and nonalcoholic steatohepatitis (NASH) in Chinese children. Therefore, this study aimed to evaluate the fecal microbiome of Chinese children with NAFLD and further analyze the potential of flora in regulating NAFLD-related symptoms and metabolic functions. Specifically, the study applied a 16S rRNA and metagenomic sequencing to the fecal samples of pediatric patients with NAFLD, NASH, and NAFL, as well as healthy controls, to explore the correlation among NAFLD-related indexes, metabolic pathways, and gut flora. The findings showed that some fecal microbiota had a negative correlation with body mass index, and various NAFLD-related bacteria, including Lachnoclostridium, Escherichia-Shigella, and Faecalibacterium prausnitzii, were detected. Consequently, the study concluded that the variation in gut microbiota might be more important in improving NAFLD/NASH compared with single species, providing a microbiota diagnostic profile of NAFLD/NASH.IMPORTANCEThis study aims to characterize the gut microbiota in Chinese children with nonalcoholic fatty liver disease (NAFLD) through 16S rRNA and metagenomic sequencing. The results highlight the association between fecal microbiota and NAFLD in Chinese children, demonstrating distinct characteristics compared to adults and children from other countries. Based on the sequencing data from our cohort's fecal samples, we propose a microbiota model with a high area under the curve for distinguishing between NAFLD and healthy individuals. Furthermore, our follow-up study reveals that changes in the relative abundance of microbial biomarkers in this model are consistent with variations in patients' body mass index. These findings suggest the potential utility of the microbiota model and microbial biomarkers for diagnosing and treating NAFLD in children.
Keywords: BMI; NAFLD; NASH; children; gut microbiota; metabolism.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



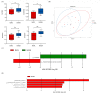
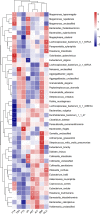
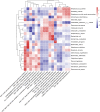
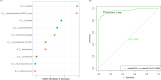

Similar articles
-
Gut Microbiota Dysbiosis in Patients with Biopsy-Proven Nonalcoholic Fatty Liver Disease: A Cross-Sectional Study in Taiwan.Nutrients. 2020 Mar 19;12(3):820. doi: 10.3390/nu12030820. Nutrients. 2020. PMID: 32204538 Free PMC article.
-
Microbiome Signatures Associated With Steatohepatitis and Moderate to Severe Fibrosis in Children With Nonalcoholic Fatty Liver Disease.Gastroenterology. 2019 Oct;157(4):1109-1122. doi: 10.1053/j.gastro.2019.06.028. Epub 2019 Jun 27. Gastroenterology. 2019. PMID: 31255652 Free PMC article.
-
Longitudinal 16S rRNA Sequencing Reveals Relationships among Alterations of Gut Microbiota and Nonalcoholic Fatty Liver Disease Progression in Mice.Microbiol Spectr. 2022 Jun 29;10(3):e0004722. doi: 10.1128/spectrum.00047-22. Epub 2022 Jun 1. Microbiol Spectr. 2022. PMID: 35647690 Free PMC article.
-
Understanding the Role of the Gut Microbiome and Microbial Metabolites in Non-Alcoholic Fatty Liver Disease: Current Evidence and Perspectives.Biomolecules. 2021 Dec 31;12(1):56. doi: 10.3390/biom12010056. Biomolecules. 2021. PMID: 35053205 Free PMC article. Review.
-
The role of the microbiome in NAFLD and NASH.EMBO Mol Med. 2019 Feb;11(2):e9302. doi: 10.15252/emmm.201809302. EMBO Mol Med. 2019. PMID: 30591521 Free PMC article. Review.
Cited by
-
Meta-analysis of shotgun sequencing of gut microbiota in obese children with MASLD or MASH.Gut Microbes. 2025 Dec;17(1):2508951. doi: 10.1080/19490976.2025.2508951. Epub 2025 May 21. Gut Microbes. 2025. PMID: 40396204 Free PMC article.
-
Development and validation of a multimodal model integrating gut microbiota and metabolite for identifying sarcopenia in patients with MASLD: a study from two centers in China.Nutr J. 2025 Aug 22;24(1):129. doi: 10.1186/s12937-025-01198-2. Nutr J. 2025. PMID: 40847308 Free PMC article.
References
-
- Adams LA, Wang Z, Liddle C, Melton PE, Ariff A, Chandraratna H, Tan J, Ching H, Coulter S, de Boer B, Christophersen CT, O’Sullivan TA, Morrison M, Jeffrey GP. 2020. Bile acids associate with specific gut microbiota, low‐level alcohol consumption and liver fibrosis in patients with non‐alcoholic fatty liver disease. Liver Int 40:1356–1365. doi:10.1111/liv.14453 - DOI - PubMed
-
- Boursier J, Mueller O, Barret M, Machado M, Fizanne L, Araujo-Perez F, Guy CD, Seed PC, Rawls JF, David LA, Hunault G, Oberti F, Calès P, Diehl AM. 2016. The severity of nonalcoholic fatty liver disease is associated with gut dysbiosis and shift in the metabolic function of the gut microbiota. Hepatology 63:764–775. doi:10.1002/hep.28356 - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
- XXT22/Digestive Medical Coordinated Development Center of Beijing Hospitals Authority
- 7222060/Beijing Natural Science Foundation
- 2020JH2/10300041/Liaoning Natural Science Foundation
- DFL20221003/Beijing Hospitals Authority's Ascent Plan
- LC2021A06/Beijing Hope Run Special Fund of Cancer Foundation of China
LinkOut - more resources
Full Text Sources
Medical

