Factors Associated with Symptom Stabilization that Allow for Successful Transition from Once-Monthly Paliperidone Palmitate to Three-Monthly Paliperidone Palmitate: A Post Hoc Analysis Examined Clinical Characteristics in Chinese Patients with Schizophrenia
- PMID: 38190077
- PMCID: PMC10810987
- DOI: 10.1007/s40263-023-01056-x
Factors Associated with Symptom Stabilization that Allow for Successful Transition from Once-Monthly Paliperidone Palmitate to Three-Monthly Paliperidone Palmitate: A Post Hoc Analysis Examined Clinical Characteristics in Chinese Patients with Schizophrenia
Abstract
Background and objectives: Identifying key factors for a successful transition from once-monthly paliperidone palmitate (PP1M) to three-monthly paliperidone palmitate (PP3M) is crucial for improving treatment outcomes, enhancing patient adherence, and reducing relapse risk in patients with schizophrenia. Providing region-specific insights for evidence-based clinical decisions can aid clinicians in optimizing transition strategies for Chinese patients with schizophrenia. Therefore, the objective of this post hoc analysis of a double-blind parallel-group multicenter phase 3 study (NCT01515423) was to identify factors related to the disease stabilization that may allow for a successful transition from PP1M to PP3M in the treatment of Chinese patients with schizophrenia.
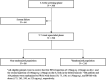
Methods: Adults (18-70 years) diagnosed with schizophrenia using the Diagnostic and Statistical Manual of Mental Disorders, fourth edition text revision, for over 1 year and with a baseline Positive and Negative Syndrome Scale (PANSS) total score between 70 and 120 were entered into an open-label (OL) phase receiving PP1M for 17 weeks. After the 17-week OL phase, patients who met the criteria necessary for stabilization were randomized (1:1) to PP1M (fixed-dose, 50, 75, 100, or 150 mg eq.) or PP3M (fixed-dose, 175, 263, 350, or 525 mg eq.) in a 48-week double-blind phase. Stabilization was defined as a PANSS total score < 70, PANSS item (P1, P2, P3, P6, P7, G8, G14) scores ≤ 4, and a reduction in Clinical Global Impression Severity (CGI-S) score of ≥ 1 from OL baseline. This post hoc analysis evaluated changes and trends in symptom severity using PANSS, changes in mental states using CGI-S, and changes in personal and social functioning using Personal and Social Performance (PSP) scores from baseline to the endpoint of the OL phase in patients who either met or did not meet the stabilization criteria (stabilized versus non-stabilized group). Comparison of changes and trends in the clinical scores between the stabilized group and non-stabilized group were conducted using linear mixed model and Mann-Kendall trend analysis, respectively. Univariate and multivariate logistic regression analyses were conducted to explore factors associated with stabilization status for transition.
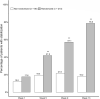
Results: Of 296 patients enrolled, 210 achieved disease stabilization (106 patients and 104 patients were randomized to PP1M and PP3M, respectively). Significant downward trends in the PANSS and CGI-S scores were detected in the stabilized patients (n = 210, ZPANSS = -2.21, p = 0.028; ZCGI-S = -2.21, p = 0.028) but not in the non-stabilized patients (n = 86). No significant trends in the PSP scores were observed in either group. The factors significantly associated with disease stabilization were the CGI-S score at baseline [odds ratio (OR) = 0.22, 95% confidence interval (CI): 0.09, 0.5), reduction of the PANSS score at week 13 (OR = 1.11, 95% CI: 1.06, 1.17), and reduction of CGI-S score at week 13 (OR = 2.27, 95% CI: 1.03, 5.02).
Conclusion: A lower CGI-S total score at baseline and greater reductions in PANSS and CGI-S scores at week 13 were associated with patients achieving disease stabilization, that may allow for a successful transition. Evidence from this study indicates that better disease condition at baseline, early functional improvement and symptomatic relief were the key factors associated with disease stabilization. The findings may guide clinicians to identify suitable patients for transition from PP1M to PP3M and further optimize the use of PP3M in China.
Clinical trials registration: EudraCT number: 2011-004889-15 and ClinicalTrials.gov (identifier: NCT01515423) for the original double-blind randomized study.
© 2024. The Author(s).
Conflict of interest statement
Gang Wang reports receiving grants outside of the submitted work from Xi'an Janssen, Eli Lily, GlaxoSmithKline, Dainippon Sumitomo Pharma, Otsuka Pharmaceutical, and ShiJiaZhuang No 4 Pharm. Shandong Luye, Chiatai Tianqing, and Jiangsu Hansoh. Xin Li, Chong Ye, Wangyi Zhang, and Miaomiao Jia are employees of Xi’an Janssen Pharmaceutical Ltd.
Figures

Similar articles
-
Paliperidone Palmitate 3-Monthly Versus 1-Monthly Injectable in Patients With Schizophrenia With or Without Prior Exposure to Oral Risperidone or Paliperidone: A Post Hoc, Subgroup Analysis.Clin Drug Investig. 2018 Aug;38(8):695-702. doi: 10.1007/s40261-018-0647-z. Clin Drug Investig. 2018. PMID: 29882073 Clinical Trial.
-
Comparing two transitioning strategies to paliperidone palmitate once-every-6-months.CNS Spectr. 2024 Dec;29(6):633-639. doi: 10.1017/S1092852924000476. Epub 2024 Nov 8. CNS Spectr. 2024. PMID: 39511777 Clinical Trial.
-
Defining "Adequately Treated": A Post Hoc Analysis Examining Characteristics of Patients with Schizophrenia Successfully Transitioned from Once-Monthly Paliperidone Palmitate to Once-Every-3-Months Paliperidone Palmitate.Neuropsychiatr Dis Treat. 2021 Jan 6;17:1-9. doi: 10.2147/NDT.S278298. eCollection 2021. Neuropsychiatr Dis Treat. 2021. PMID: 33442251 Free PMC article.
-
Characteristics of patients with schizophrenia switching from oral antipsychotics to once-monthly paliperidone palmitate (PP1M): a systematic review.BMC Psychiatry. 2024 Jan 19;24(1):57. doi: 10.1186/s12888-024-05508-6. BMC Psychiatry. 2024. PMID: 38243208 Free PMC article.
-
Effectiveness and Safety of Switching from Oral Antipsychotics to Once-Monthly Paliperidone Palmitate (PP1M) in the Management of Schizophrenia: A Systematic Review and Meta-Analysis.CNS Drugs. 2023 Aug;37(8):695-713. doi: 10.1007/s40263-023-01028-1. Epub 2023 Jul 25. CNS Drugs. 2023. PMID: 37490267 Free PMC article.
Cited by
-
A multidimensional assessment of adverse events associated with paliperidone palmitate: a real-world pharmacovigilance study using the FAERS and JADER databases.BMC Psychiatry. 2025 Jan 20;25(1):52. doi: 10.1186/s12888-025-06493-0. BMC Psychiatry. 2025. PMID: 39833706 Free PMC article.
-
Health-Care Utilisation and Costs of Transition from Paliperidone Palmitate 1-Monthly to 3-Monthly Treatment for Schizophrenia: A Real-World, Retrospective, 24-Month Mirror-Image Study.Neuropsychiatr Dis Treat. 2024 Oct 19;20:1985-1993. doi: 10.2147/NDT.S484717. eCollection 2024. Neuropsychiatr Dis Treat. 2024. PMID: 39450244 Free PMC article.
References
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

