Argatroban in Patients With Acute Ischemic Stroke With Early Neurological Deterioration: A Randomized Clinical Trial
- PMID: 38190136
- PMCID: PMC10775075
- DOI: 10.1001/jamaneurol.2023.5093
Argatroban in Patients With Acute Ischemic Stroke With Early Neurological Deterioration: A Randomized Clinical Trial
Abstract
Importance: The effect of argatroban in patients with acute ischemic stroke (AIS) and early neurological deterioration (END) is unknown.
Objective: To assess the efficacy of argatroban for END in AIS.
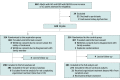
Design, setting, and participants: This open-label, blinded-end point, randomized clinical trial was conducted from April 4, 2020, through July 31, 2022. The date of final follow-up was October 31, 2022. This was a multicenter trial. Eligible patients were adults with AIS who experienced END, which was defined as an increase of 2 or more points on the National Institutes of Health Stroke Scale within 48 hours from symptom onset. Patients who withdrew consent, experienced duplicate randomization, or were lost to follow-up were excluded from the study.
Interventions: Patients were randomly assigned to the argatroban group and control group within 48 hours of symptom onset. Both groups received standard therapy based on guidelines, including oral mono or dual antiplatelet therapy. The argatroban group received intravenous argatroban for 7 days (continuous infusion at a dose of 60 mg per day for 2 days, followed by 20 mg per day for 5 days) in addition to standard therapy.
Main outcome and measure: The primary end point was good functional outcome at 90 days, defined as a modified Rankin Scale score of 0 to 3.
Results: A total of 628 patients (mean [SD] age, 65 [11.9] years; 400 male [63.7%]) were included in this study (argatroban group, 314 [50%] and control group, 314 [50%]). Of these, 18 withdrew consent, 1 had duplicate randomization, and 8 were lost to follow-up. A total of 601 patients with stroke were included in the intention-to-treat analysis. Finally, 564 patients were included in the per-protocol analysis as 6 participants in the argatroban group and 31 participants in the control group did not follow the complete protocol. The number of patients with good functional outcome at 90 days was 240 (80.5%) in the argatroban group and 222 (73.3%) in the control group (risk difference, 7.2%; 95% CI, 0.6%-14.0%; risk ratio, 1.10; 95% CI, 1.01-1.20; P = .04). The proportion of symptomatic intracranial hemorrhage was 3 of 317 (0.9%) in the argatroban group and 2 of 272 (0.7%) in the control group (P = .78).
Conclusions and relevance: Among patients with AIS with END, treatment with argatroban and antiplatelet therapy resulted in a better functional outcome at 90 days. This trial provided evidence to support the use of argatroban in reducing disability for patients with END.
Trial registration: ClinicalTrials.gov Identifier: NCT04275180.
Conflict of interest statement
Figures

Comment in
-
The Case of Anticoagulation for Progressing Stroke: Have We Come Full Circle?JAMA Neurol. 2024 Feb 1;81(2):113-114. doi: 10.1001/jamaneurol.2023.5086. JAMA Neurol. 2024. PMID: 38190153 No abstract available.
References
-
- Seners P, Turc G, Oppenheim C, Baron JC. Incidence, causes, and predictors of neurological deterioration occurring within 24 h following acute ischaemic stroke: a systematic review with pathophysiological implications. J Neurol Neurosurg Psychiatry. 2015;86(1):87-94. doi:10.1136/jnnp-2014-308327 - DOI - PubMed
-
- De Schryver EL, Algra A, Kappelle LJ, van Gijn J, Koudstaal PJ. Vitamin K antagonists vs antiplatelet therapy after transient ischaemic attack or minor ischaemic stroke of presumed arterial origin. Cochrane Database Syst Rev. 2012;2012(9):CD001342. doi:10.1002/14651858.CD001342.pub3 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

