Borataalkenes, boraalkenes, and the η2-B,C coordination mode in coordination chemistry and catalysis
- PMID: 38190152
- PMCID: PMC10922737
- DOI: 10.1039/d3cs00730h
Borataalkenes, boraalkenes, and the η2-B,C coordination mode in coordination chemistry and catalysis
Abstract
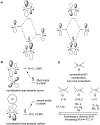
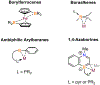
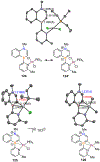
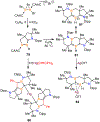
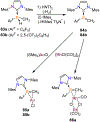
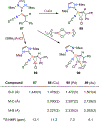
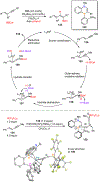
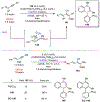
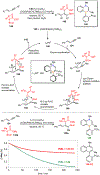
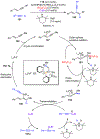
Borataalkenes and boraalkenes are the boron-containing isoelectronic analogues of alkenes and vinyl cations respectively. Compared with alkenes, the borataalkene and boraalkene ligand motifs in transition metal coordination chemistry are relatively underexplored. In this review, the synthesis of borataalkene and boraalkene complexes and other transition metal complexes featuring the η2-B,C coordination mode is described. The diversity of coordination modes and geometry in these complexes, and the spectroscopic and structural evidence supporting their assignments is disclosed as well as computational analysis of bonding. The applications of the borataalkene ligand motif in synthetic organic homogeneous catalysis, especially those involving geminal bis(pinacolatoboronates) and 1,4-azaborines, are discussed.
Conflict of interest statement
Conflicts of interest
There are no conflicts to declare.
Figures
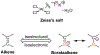




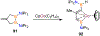

References
-
- Fairlamb J, Organic & biomolecular chemistry, 2008, 6, 3645–3656. - PubMed
- Crabtree RH, The organometallic chemistry of the transition metals, John Wiley & Sons, 2009.
- Elschenbroich C, Organometallics, John Wiley & Sons, 2016.
- Albano VG, Natile G and Panunzi A, Coord. Chem. Rev, 1994, 133, 67–114.
-
- Zeise WC, Overs. K. Dan. Vidensk. Selsk. Forth 1825-26, 13;
- Zeise WC Ann. Phys. Chem 1831, 21, 497–541.
-
- Kohrt S, Dachwitz S, Daniliuc CG, Kehr G and Erker G, Dalton Trans, 2015, 44, 21032–21040. - PubMed
- Olmstead MM, Power PP, Weese KJ and Doedens RJ, J. Am. Chem. Soc, 1987, 109, 2541–2542.
- Rathke MW and Kow R, J. Am. Chem. Soc, 1972, 94, 6854–6856.
- Cainelli G, Dal Bello G and Zubiani G, Tetrahedron Lett, 1966, 7, 4315–4318.
- Kow R and Rathke MW, J. Am. Chem. Soc, 1973, 95, 2715–2716.
- Cuenca AB and Fernández E, Chem. Soc. Rev, 2021, 50, 72–86. - PubMed
- Ramsey BG and Isabelle LM, J. Org. Chem, 1981, 46, 179–182.
- Klusik H and Berndt A, Angew. Chem., Int. Ed. Engl, 1983, 22, 877–878.
-
- Chiu C and Gabbaï FP, Angew. Chem. Int. Ed, 2007, 46, 6878–6881. - PubMed
- Hoefelmeyer JD, Solé S and Gabbaï FP, Dalton Trans, 2004, 1254–1258. - PubMed
- Wang L, Jian Z, Daniliuc CG, Kehr G and Erker G, Dalton Trans, 2018, 47, 10853–10856. - PubMed
- Möbus J, Kehr G, Daniliuc CG, Fröhlich R and Erker G, Dalton Trans, 2014, 43, 632–638. - PubMed
- Yu J, Kehr G, Daniliuc CG and Erker G, Eur. J. Inorg. Chem, 2013, 2013, 3312–3315. - PubMed
-
- Emslie DJ, Cowie BE and Kolpin KB, Dalton Trans, 2012, 41, 1101–1117. - PubMed
- Goettel JT and Braunschweig H, Coord. Chem. Rev, 2019, 380, 184–200.
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

