NALIRIFOX, FOLFIRINOX, and Gemcitabine With Nab-Paclitaxel as First-Line Chemotherapy for Metastatic Pancreatic Cancer: A Systematic Review and Meta-Analysis
- PMID: 38190183
- PMCID: PMC10774994
- DOI: 10.1001/jamanetworkopen.2023.50756
NALIRIFOX, FOLFIRINOX, and Gemcitabine With Nab-Paclitaxel as First-Line Chemotherapy for Metastatic Pancreatic Cancer: A Systematic Review and Meta-Analysis
Abstract
Importance: The NAPOLI 3 trial showed the superiority of fluorouracil, leucovorin, liposomal irinotecan, and oxaliplatin (NALIRIFOX) over the combination of gemcitabine and nab-paclitaxel (GEM-NABP) as first-line treatment of metastatic pancreatic ductal adenocarcinoma (PDAC). Analyses comparing NALIRIFOX and GEM-NABP with fluorouracil, leucovorin, irinotecan, and oxaliplatin (FOLFIRINOX) have not yet been reported.
Objective: To derive survival, response, and toxic effects data from phase 3 clinical trials and compare NALIRIFOX, FOLFIRINOX, and GEM-NABP.
Data sources: After a systematic search of PubMed, Scopus, Embase, and American Society of Clinical Oncology and European Society for Medical Oncology meetings' libraries, Kaplan-Meier curves were extracted from phase 3 clinical trials conducted from January 1, 2011, until September 12, 2023.
Study selection: Phase 3 clinical trials that tested NALIRIFOX, FOLFIRINOX, or GEM-NABP as first-line treatment of metastatic PDAC and reported overall survival (OS) and progression-free survival (PFS) curves were selected. This study followed the Preferred Reporting Items for Systematic Reviews and Meta-analyses of Individual Participant Data reporting guidelines.
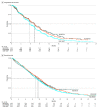
Data extraction and synthesis: Individual patient OS and PFS data were extracted from Kaplan-Meier plots of original trials via a graphic reconstructive algorithm. Overall response rates (ORRs) and grade 3 or higher toxic effects rates were also collected. A pooled analysis was conducted, and results were validated via a network meta-analysis.
Main outcomes and measures: The primary end point was OS. Secondary outcomes included PFS, ORR, and toxic effects rates.
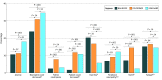
Results: A total of 7 trials with data on 2581 patients were analyzed, including 383 patients treated with NALIRIFOX, 433 patients treated with FOLFIRINOX, and 1756 patients treated with GEM-NABP. Median PFS was longer in patients treated with NALIRIFOX (7.4 [95% CI, 6.1-7.7] months) or FOLFIRINOX (7.3 [95% CI, 6.5-7.9] months; [HR], 1.21 [95% CI, 0.86-1.70]; P = .28) compared with patients treated with GEM-NABP (5.7 [95% CI, 5.6-6.1] months; HR vs NALIRIFOX, 1.45 [95% CI, 1.22-1.73]; P < .001). Similarly, GEM-NABP was associated with poorer OS (10.4 [95% CI, 9.8-10.8]; months) compared with NALIRIFOX (HR, 1.18 [95% CI, 1.00-1.39]; P = .05], while no difference was observed between FOLFIRINOX (11.7 [95% CI, 10.4-13.0] months) and NALIRIFOX (11.1 [95% CI, 10.1-12.3] months; HR, 1.06 [95% CI, 0.81-1.39]; P = .65). There were no statistically significant differences in ORR among NALIRIFOX (41.8%), FOLFIRINOX (31.6%), and GEM-NABP (35.0%). NALIRIFOX was associated with lower incidence of grade 3 or higher hematological toxic effects (eg, platelet count decreased 1.6% vs 11.8% with FOLFIRINOX and 10.8% with GEM-NABP), but higher rates of severe diarrhea compared with GEM-NABP (20.3% vs 15.7%).
Conclusions and relevance: In this systematic review and meta-analysis, NALIRIFOX and FOLFIRINOX were associated with similar PFS and OS as first-line treatment of advanced PDAC, although NALIRIFOX was associated with a different toxicity profile. Careful patient selection, financial toxic effects consideration, and direct comparison between FOLFIRINOX and NALIRIFOX are warranted.
Conflict of interest statement
Figures

References
-
- Takumoto Y, Sasahara Y, Narimatsu H, Akazawa M. Comparative outcomes of first-line chemotherapy for metastatic pancreatic cancer among the regimens used in Japan: a systematic review and network meta-analysis. JAMA Netw Open. 2022;5(1):e2145515. doi:10.1001/jamanetworkopen.2021.45515 - DOI - PMC - PubMed
-
- Wainberg ZA, Melisi D, Macarulla T, et al. . NALIRIFOX versus nab-paclitaxel and gemcitabine in treatment-naive patients with metastatic pancreatic ductal adenocarcinoma (NAPOLI 3): a randomised, open-label, phase 3 trial. Lancet. 2023;402(10409):1272-1281. doi:10.1016/S0140-6736(23)01366-1 - DOI - PMC - PubMed

