Bevacizumab, Irinotecan, or Topotecan Added to Temozolomide for Children With Relapsed and Refractory Neuroblastoma: Results of the ITCC-SIOPEN BEACON-Neuroblastoma Trial
- PMID: 38190578
- PMCID: PMC11003502
- DOI: 10.1200/JCO.23.00458
Bevacizumab, Irinotecan, or Topotecan Added to Temozolomide for Children With Relapsed and Refractory Neuroblastoma: Results of the ITCC-SIOPEN BEACON-Neuroblastoma Trial
Abstract
Purpose: Outcomes for children with relapsed and refractory high-risk neuroblastoma (RR-HRNB) remain dismal. The BEACON Neuroblastoma trial (EudraCT 2012-000072-42) evaluated three backbone chemotherapy regimens and the addition of the antiangiogenic agent bevacizumab (B).
Materials and methods: Patients age 1-21 years with RR-HRNB with adequate organ function and performance status were randomly assigned in a 3 × 2 factorial design to temozolomide (T), irinotecan-temozolomide (IT), or topotecan-temozolomide (TTo) with or without B. The primary end point was best overall response (complete or partial) rate (ORR) during the first six courses, by RECIST or International Neuroblastoma Response Criteria for patients with measurable or evaluable disease, respectively. Safety, progression-free survival (PFS), and overall survival (OS) time were secondary end points.
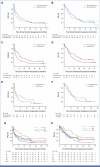
Results: One hundred sixty patients with RR-HRNB were included. For B random assignment (n = 160), the ORR was 26% (95% CI, 17 to 37) with B and 18% (95% CI, 10 to 28) without B (risk ratio [RR], 1.52 [95% CI, 0.83 to 2.77]; P = .17). Adjusted hazard ratio for PFS and OS were 0.89 (95% CI, 0.63 to 1.27) and 1.01 (95% CI, 0.70 to 1.45), respectively. For irinotecan ([I]; n = 121) and topotecan (n = 60) random assignments, RRs for ORR were 0.94 and 1.22, respectively. A potential interaction between I and B was identified. For patients in the bevacizumab-irinotecan-temozolomide (BIT) arm, the ORR was 23% (95% CI, 10 to 42), and the 1-year PFS estimate was 0.67 (95% CI, 0.47 to 0.80).
Conclusion: The addition of B met protocol-defined success criteria for ORR and appeared to improve PFS. Within this phase II trial, BIT showed signals of antitumor activity with acceptable tolerability. Future trials will confirm these results in the chemoimmunotherapy era.
Trial registration: ClinicalTrials.gov NCT02308527.
Conflict of interest statement
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (
No other potential conflicts of interest were reported.
Figures

References
-
- Moreno L, Rubie H, Varo A, et al. : Outcome of children with relapsed or refractory neuroblastoma: A meta-analysis of ITCC/SIOPEN European phase II clinical trials. Pediatr Blood Cancer 64:25-31, 2017 - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

