Two Receptor Binding Strategy of SARS-CoV-2 Is Mediated by Both the N-Terminal and Receptor-Binding Spike Domain
- PMID: 38190651
- PMCID: PMC10801686
- DOI: 10.1021/acs.jpcb.3c06258
Two Receptor Binding Strategy of SARS-CoV-2 Is Mediated by Both the N-Terminal and Receptor-Binding Spike Domain
Abstract
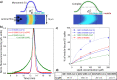
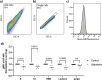
It is not well understood why severe acute respiratory syndrome (SARS)-CoV-2 spreads much faster than other β-coronaviruses such as SARS-CoV and Middle East respiratory syndrome (MERS)-CoV. In a previous publication, we predicted the binding of the N-terminal domain (NTD) of SARS-CoV-2 spike to sialic acids (SAs). Here, we experimentally validate this interaction and present simulations that reveal a second possible interaction between SAs and the spike protein via a binding site located in the receptor-binding domain (RBD). The predictions from molecular-dynamics simulations and the previously-published 2D-Zernike binding-site recognition approach were validated through flow-induced dispersion analysis (FIDA)─which reveals the capability of the SARS-CoV-2 spike to bind to SA-containing (glyco)lipid vesicles, and flow-cytometry measurements─which show that spike binding is strongly decreased upon inhibition of SA expression on the membranes of angiotensin converting enzyme-2 (ACE2)-expressing HEK cells. Our analyses reveal that the SA binding of the NTD and RBD strongly enhances the infection-inducing ACE2 binding. Altogether, our work provides in silico, in vitro, and cellular evidence that the SARS-CoV-2 virus utilizes a two-receptor (SA and ACE2) strategy. This allows the SARS-CoV-2 spike to use SA moieties on the cell membrane as a binding anchor, which increases the residence time of the virus on the cell surface and aids in the binding of the main receptor, ACE2, via 2D diffusion.
Conflict of interest statement
The authors declare no competing financial interest.
Figures



References
-
- Wang H.; Paulson K. R.; Pease S. A.; Watson S.; Comfort H.; Zheng P.; Aravkin A. Y.; Bisignano C.; Barber R. M.; Alam T.; et al. Estimating excess mortality due to the COVID-19 pandemic: a systematic analysis of COVID-19-related mortality, 2020–21. Lancet 2022, 399, 1513–1536. 10.1016/S0140-6736(21)02796-3. - DOI - PMC - PubMed
-
- Liu D. X.; Liang J. Q.; Fung T. S.. Human Coronavirus-229E, -OC43, -NL63, and -HKU1 (Coronaviridae). In Encyclopedia of Virology, 2021; pp 428–440.10.1016/B978-0-12-809633-8.21501-X. - DOI
-
- Drosten C.; Günther S.; Preiser W.; Van Der Werf S.; Brodt H.-R.; Becker S.; Rabenau H.; Panning M.; Kolesnikova L.; Fouchier R. A.; et al. Identification of a novel coronavirus in patients with severe acute respiratory syndrome. N. Engl. J. Med. 2003, 348, 1967–1976. 10.1056/NEJMoa030747. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

