Development of a Strategic Initiative at MD Anderson Cancer Center to Improve Outcomes in Immune-Related Adverse Events
- PMID: 38190801
- PMCID: PMC12381944
- DOI: 10.6004/jnccn.2023.7119
Development of a Strategic Initiative at MD Anderson Cancer Center to Improve Outcomes in Immune-Related Adverse Events
Abstract
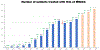
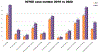
Immune checkpoint inhibitors (ICIs) have transformed the treatment paradigm for many cancer types. The clinical use of ICIs is increasing rapidly, including in combinations associated with increased risk of toxicities, termed "immune-related adverse events" (irAEs). Therefore, MD Anderson Cancer Center (MDACC) in Houston, Texas has proactively responded by developing a priority endeavor known as the Immuno-Oncology Toxicity (IOTOX) initiative. This strategic initiative aims to facilitate the seamless integration of key domains: (1) standardized clinical practice and innovative decision toolsets; (2) patient and provider education; and (3) a comprehensive clinical and translational research platform. The ultimate goal of this initiative is to develop and disseminate clinical best practices and biologic insights into irAEs to improve outcomes of patients with irAEs at MDACC and in the wider oncology community.
Figures

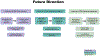
References
-
- Sharma P, et al. , The Next Decade of Immune Checkpoint Therapy. Cancer Discov, 2021. 11(4): p. 838–857. - PubMed
-
- Schneider BJ, et al. , Management of Immune-Related Adverse Events in Patients Treated With Immune Checkpoint Inhibitor Therapy: ASCO Guideline Update. J Clin Oncol, 2021. 39(36): p. 4073–4126. - PubMed
-
- Thompson JA, et al. , Management of Immunotherapy-Related Toxicities, Version 1.2022, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw, 2022. 20(4): p. 387–405. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

