Efficacy, safety, and pharmacokinetics of capsid assembly modulator linvencorvir plus standard of care in chronic hepatitis B patients
- PMID: 38190830
- PMCID: PMC11016473
- DOI: 10.3350/cmh.2023.0422
Efficacy, safety, and pharmacokinetics of capsid assembly modulator linvencorvir plus standard of care in chronic hepatitis B patients
Abstract
Background/aims: Four-week treatment of linvencorvir (RO7049389) was generally safe and well tolerated, and showed anti-viral activity in chronic hepatitis B (CHB) patients. This study evaluated the efficacy, safety, and pharmacokinetics of 48-week treatment with linvencorvir plus standard of care (SoC) in CHB patients.
Methods: This was a multicentre, non-randomized, non-controlled, open-label phase 2 study enrolling three cohorts: nucleos(t)ide analogue (NUC)-suppressed patients received linvencorvir plus NUC (Cohort A, n=32); treatment-naïve patients received linvencorvir plus NUC without (Cohort B, n=10) or with (Cohort C, n=30) pegylated interferon-α (Peg-IFN-α). Treatment duration was 48 weeks, followed by NUC alone for 24 weeks.
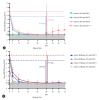
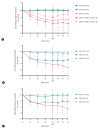
Results: 68 patients completed the study. No patient achieved functional cure (sustained HBsAg loss and unquantifiable HBV DNA). By Week 48, 89% of treatment-naïve patients (10/10 Cohort B; 24/28 Cohort C) reached unquantifiable HBV DNA. Unquantifiable HBV RNA was achieved in 92% of patients with quantifiable baseline HBV RNA (14/15 Cohort A, 8/8 Cohort B, 22/25 Cohort C) at Week 48 along with partially sustained HBV RNA responses in treatment-naïve patients during follow-up period. Pronounced reductions in HBeAg and HBcrAg were observed in treatment-naïve patients, while HBsAg decline was only observed in Cohort C. Most adverse events were grade 1-2, and no linvencorvir-related serious adverse events were reported.
Conclusion: 48-week linvencorvir plus SoC was generally safe and well tolerated, and resulted in potent HBV DNA and RNA suppression. However, 48-week linvencorvir plus NUC with or without Peg-IFN did not result in the achievement of functional cure in any patient.
Keywords: Capsid assembly modulator; Chronic hepatitis B; Linvencorvir; Phase 2; RO7049389.
Conflict of interest statement
Jinlin Hou received grants from Bristol Myers Squibb and Johnson & Johnson; and declared other financial or non-financial interests with AbbVie, Arbutus, Bristol Myers Squibb, Gilead Sciences, Johnson & Johnson, and Hoffmann-La Roche.
Edward Gane is an advisory committee or review panel member for AbbVie, Arbutus, Arrowhead, Assembly Biosciences, Availa, Clear B Therapeutics, Dicerna, Finch Therapeutics, Gilead Sciences, Janssen, Novartis, Hoffmann-La Roche, and Vir Bio; and has received speaking and teaching fees from AbbVie, Aligos, DrugFarm, Enanta, Gilead, GlaxoSmithKline, Janssen, Merck, and Novartis.
Sheng-Shun Yang has received speaking fees from AbbVie, Bristol-Myers Squibb, Gilead Sciences, Ipsen, and Merck Sharp & Dohme, and served as an advisory board member for AbbVie, Gilead Sciences, Hoffmann-La Roche, Sysmex, and Ipsen.
Seng Gee Lim received payment or honoraria for lectures from Gilead, Janssen, Hoffman-La Roche, Sysmex and GSK; served as advisory board member for Gliead, Abbot, Hoffmann-La Roche, GSK, Janssen, Sysmex, Abrutus, Assembly, and Grifols; served in leadership role in ICE-HBV, HBV Forum, AASLD Asia Pacific Advisory Board, and ANRS-MIE Advisory Board; and receipt of research support from Gilead, Abbot, Hoffmann-LaRoche, Sysmex, Fibronostics, and Merck.
Man-Fung Yuen serves as advisory board member/ consultant for and/or received research funding from AbbVie, Aligos Therarpeutics, AiCuris, Antios Therapeutics, Arrowhead Pharmaceuticals, Arbutus Biopharma, Assembly Biosciences, Bristol Myer Squibb, Bluejay Therapeutics, Clear B Therapeutics, Dicerna Pharmaceuticals, Finch Therapeutics, Fujirebio Incorporation, GlaxoSmithKline, Gilead Sciences, Immunocore, Janssen, Merck Sharp and Dohme, and Hoffmann-La Roche, Vir Biotechnology.
Rozalina Balabanska, Wenhong Zhang, Jiming Zhang, Tien Huey Lim, Qing Xie, Chau-Ting Yeh, Xieer Liang, Piyawat Komolmit, Apinya Leerapun, Ting-Tsung Chang, Tsung-Hui Hu, Wan-Long Chuang, and Barbara Leggett declare no competing interests.
Zenghui Xue, Ethan Chen, Yuchen Zhang, Qiaoqiao Xie and Wen Zhang are employees of Hoffmann-La Roche. Qingyan Bo and Xue Zhou were former employees of Hoffmann-La Roche.
Figures




Comment in
-
Linvencovir: Paving the way for functional cure in hepatitis B.Clin Mol Hepatol. 2024 Apr;30(2):164-165. doi: 10.3350/cmh.2024.0149. Epub 2024 Mar 6. Clin Mol Hepatol. 2024. PMID: 38447533 Free PMC article. No abstract available.
References
-
- World Health Organization (WHO) Hepatitis B. WHO Fact sheets. WHO web site, < https://www.who.int/news-room/fact-sheets/detail/hepatitis-b>. Accessed 18 Jul 2023.
-
- Lok AS, Zoulim F, Dusheiko G, Ghany MG. Hepatitis B cure: From discovery to regulatory approval. J Hepatol. 2017;67:847–861. - PubMed
-
- Anderson RT, Choi HSJ, Lenz O, Peters MG, Janssen HLA, Mishra P, et al. Association between seroclearance of hepatitis B surface antigen and long-term clinical outcomes of patients with chronic hepatitis B virus infection: Systematic review and meta-analysis. Clin Gastroenterol Hepatol. 2021;19:463–472. - PubMed
-
- European Association for the Study of the Liver EASL 2017 Clinical Practice Guidelines on the management of hepatitis B virus infection. J Hepatol. 2017;67:370–398. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- Antios Therapeutics
- Arrowhead Pharmaceuticals
- Arbutus Biopharma
- Assembly Biosciences
- Bluejay Therapeutics
- Clear B Therapeutics
- Dicerna Pharmaceuticals
- Finch Therapeutics
- Fujirebio Incorporation
- GlaxoSmithKline
- Gilead Sciences
- Immunocore
- Janssen
- Merck Sharp and Dohme
- Hoffmann-La Roche
- Vir Biotechnology
LinkOut - more resources
Full Text Sources
Miscellaneous

