Clinically relevant orthotopic pancreatic cancer models for adoptive T cell transfer therapy
- PMID: 38191243
- PMCID: PMC10806555
- DOI: 10.1136/jitc-2023-008086
Clinically relevant orthotopic pancreatic cancer models for adoptive T cell transfer therapy
Abstract
Background: Pancreatic ductal adenocarcinoma (PDAC) is an aggressive tumor. Prognosis is poor and survival is low in patients diagnosed with this disease, with a survival rate of ~12% at 5 years. Immunotherapy, including adoptive T cell transfer therapy, has not impacted the outcomes in patients with PDAC, due in part to the hostile tumor microenvironment (TME) which limits T cell trafficking and persistence. We posit that murine models serve as useful tools to study the fate of T cell therapy. Currently, genetically engineered mouse models (GEMMs) for PDAC are considered a "gold-standard" as they recapitulate many aspects of human disease. However, these models have limitations, including marked tumor variability across individual mice and the cost of colony maintenance.
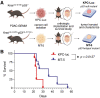
Methods: Using flow cytometry and immunohistochemistry, we characterized the immunological features and trafficking patterns of adoptively transferred T cells in orthotopic PDAC (C57BL/6) models using two mouse cell lines, KPC-Luc and MT-5, isolated from C57BL/6 KPC-GEMM (KrasLSL-G12D/+p53-/- and KrasLSL-G12D/+p53LSL-R172H/+, respectively).
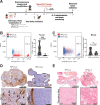
Results: The MT-5 orthotopic model best recapitulates the cellular and stromal features of the TME in the PDAC GEMM. In contrast, far more host immune cells infiltrate the KPC-Luc tumors, which have less stroma, although CD4+ and CD8+ T cells were similarly detected in the MT-5 tumors compared with KPC-GEMM in mice. Interestingly, we found that chimeric antigen receptor (CAR) T cells redirected to recognize mesothelin on these tumors that signal via CD3ζ and 41BB (Meso-41BBζ-CAR T cells) infiltrated the tumors of mice bearing stroma-devoid KPC-Luc orthotopic tumors, but not MT-5 tumors.
Conclusions: Our data establish for the first time a reproducible and realistic clinical system useful for modeling stroma-rich and stroma-devoid PDAC tumors. These models shall serve an indepth study of how to overcome barriers that limit antitumor activity of adoptively transferred T cells.
Keywords: CD4-CD8 ratio; T-lymphocytes; receptors, chimeric antigen; receptors, immunologic; tumor microenvironment.
© Author(s) (or their employer(s)) 2024. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: GBL has consulted for ProDa Biotech and received compensation. GBL has also received research funding through a sponsored research agreement between Emory University and Merck and Co, Bristol-Myers Squibb, Boehringer Ingelheim, and Vaccinex. CP has received research funding through a sponsored research agreement between the Medical University of South Carolina and Obsidian, Lycera, and ThermoFisher, and is the cofounder of Ares Immunotherapy.
Figures

References
-
- Cancer facts & figures. American Cancer Society, Available: https://www.cancer.org/research/cancer-facts-statistics/all-cancer-facts...
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous
