Establishment of a prognostic risk prediction model incorporating disulfidptosis-related lncRNA for patients with prostate cancer
- PMID: 38191330
- PMCID: PMC10775669
- DOI: 10.1186/s12885-023-11778-2
Establishment of a prognostic risk prediction model incorporating disulfidptosis-related lncRNA for patients with prostate cancer
Abstract
Purpose: Prostate cancer (PCa) is one of the major tumor diseases that threaten men's health globally, and biochemical recurrence significantly impacts its prognosis. Disulfidptosis, a recently discovered cell death mechanism triggered by intracellular disulfide accumulation leading to membrane rupture, is a new area of research in the context of PCa. Currently, its impact on PCa remains largely unexplored. This study aims to investigate the correlation between long non-coding RNAs (lncRNAs) associated with disulfidptosis and the prognosis of PCa, seeking potential connections between the two.
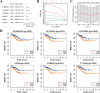
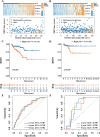
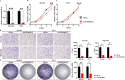
Methods: Transcriptomic data for a PCa cohort were obtained from the Cancer Genome Atlas database. Disulfidptosis-related lncRNAs (DDRLs) were identified through differential expression and Pearson correlation analysis. DDRLs associated with biochemical recurrence-free survival (BRFS) were precisely identified using univariate Cox and LASSO regression, resulting in the development of a risk score model. Clinical factors linked to BRFS were determined through both univariate and multivariate Cox analyses. A prognostic nomogram combined the risk score with key clinical variables. Model performance was assessed using Receiver Operating Characteristic (ROC) curves, Decision Curve Analysis (DCA), and calibration curves. The functional impact of a critical DDRL was substantiated through assays involving CCK8, invasion, migration, and cell cloning. Additionally, immunohistochemical (IHC) staining for the disulfidptosis-related protein SLC7A11 was conducted.
Results: The prognostic signature included AC026401.3, SNHG4, SNHG25, and U73166.1 as key components. The derived risk score from these signatures stood as one of the independent prognostic factor for PCa patients, correlating with poorer BRFS in the high-risk group. By combining the risk score with clinical variables, a practical nomogram was created, accurately predicting BRFS of PCa patients. Notably, silencing AC026401.3 significantly hindered PCa cell proliferation, invasion, migration, and colony formation. IHC staining revealed elevated expression of the dithiosulfatide-related protein SLC7A11 in tumor tissue.
Conclusions: A novel prognostic signature for PCa DDRLs, possessing commendable predictive power, has been constructed, simultaneously providing potential therapeutic targets associated with disulfidptosis, among which AC026401.3 has been validated in vitro and demonstrated inhibition of PCa tumorigenesis after its silencing.
Keywords: AC026401.3; Biochemical recurrence-free survival; Disulfidptosis; Prostate cancer; lncRNA.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures



Similar articles
-
Development of a novel disulfidptosis-related lncRNA signature for prognostic and immune response prediction in clear cell renal cell carcinoma.Sci Rep. 2024 Jan 5;14(1):624. doi: 10.1038/s41598-024-51197-2. Sci Rep. 2024. PMID: 38182642 Free PMC article.
-
Bioinformatics-based analysis of the relationship between disulfidptosis and prognosis and treatment response in pancreatic cancer.Sci Rep. 2023 Dec 14;13(1):22218. doi: 10.1038/s41598-023-49752-4. Sci Rep. 2023. PMID: 38097783 Free PMC article. Clinical Trial.
-
Integrated Bioinformatics and Experimental Validation to Identify a Disulfidptosis-Related lncRNA Model for Prognostic Prediction in Papillary Renal Cell Carcinoma.Comb Chem High Throughput Screen. 2025;28(5):883-898. doi: 10.2174/0113862073303084240403051346. Comb Chem High Throughput Screen. 2025. PMID: 38639274
-
Therapeutic Potential of lncRNAs in Regulating Disulfidptosis for Cancer Treatment.Pathol Res Pract. 2024 Nov;263:155657. doi: 10.1016/j.prp.2024.155657. Epub 2024 Oct 16. Pathol Res Pract. 2024. PMID: 39437641 Review.
-
Disulfidptosis: a novel cell death modality induced by actin cytoskeleton collapse and a promising target for cancer therapeutics.Cell Commun Signal. 2024 Oct 11;22(1):491. doi: 10.1186/s12964-024-01871-9. Cell Commun Signal. 2024. PMID: 39394612 Free PMC article. Review.
Cited by
-
Prognostic value of disulfidptosis-associated genes in gastric cancer: a comprehensive analysis.Front Oncol. 2025 Mar 4;15:1512394. doi: 10.3389/fonc.2025.1512394. eCollection 2025. Front Oncol. 2025. PMID: 40104507 Free PMC article.
-
Multi‑cohort Validation Based on Disulfidptosis-Related lncRNAs for Predicting Prognosis and Immunotherapy Response of Esophageal Squamous Cell Carcinoma.Onco Targets Ther. 2025 Jun 25;18:763-778. doi: 10.2147/OTT.S519270. eCollection 2025. Onco Targets Ther. 2025. PMID: 40589861 Free PMC article.
-
Disulfidptosis in tumor progression.Cell Death Discov. 2025 Apr 28;11(1):205. doi: 10.1038/s41420-025-02495-9. Cell Death Discov. 2025. PMID: 40295497 Free PMC article. Review.
-
Integrated analysis and experiments uncover the function of disulfidptosis in predicting immunotherapy effectiveness and delineating immune landscapes in uterine corpus endometrial carcinoma.Front Immunol. 2024 Oct 9;15:1454730. doi: 10.3389/fimmu.2024.1454730. eCollection 2024. Front Immunol. 2024. PMID: 39445012 Free PMC article.
-
Utilizing Liquid-liquid phase separation-related lncRNAs to predict the prognosis and treatment response of PCa.Discov Oncol. 2024 Aug 16;15(1):352. doi: 10.1007/s12672-024-01226-3. Discov Oncol. 2024. PMID: 39150479 Free PMC article.
References
MeSH terms
Substances
Grants and funding
- 2023B1111030006/Guangdong Province key areas research and development plan
- 2023B1111030006/Guangdong Province key areas research and development plan
- 2023B1111030006/Guangdong Province key areas research and development plan
- 2023B1111030006/Guangdong Province key areas research and development plan
- 2023B1111030006/Guangdong Province key areas research and development plan
- 2023B1111030006/Guangdong Province key areas research and development plan
- 2023B1111030006/Guangdong Province key areas research and development plan
- 2023B1111030006/Guangdong Province key areas research and development plan
- 2023B1111030006/Guangdong Province key areas research and development plan
- 82072841/National Natural Science Foundation of China
- 82072841/National Natural Science Foundation of China
- 82072841/National Natural Science Foundation of China
- 82072841/National Natural Science Foundation of China
- 82072841/National Natural Science Foundation of China
- 82072841/National Natural Science Foundation of China
- 82072841/National Natural Science Foundation of China
- 82072841/National Natural Science Foundation of China
- 82072841/National Natural Science Foundation of China
- 2021A1515010199/Natural Science Foundation of Guangdong Province
- 2021A1515010199/Natural Science Foundation of Guangdong Province
- 2021A1515010199/Natural Science Foundation of Guangdong Province
- 2021A1515010199/Natural Science Foundation of Guangdong Province
- 2021A1515010199/Natural Science Foundation of Guangdong Province
- 2021A1515010199/Natural Science Foundation of Guangdong Province
- 2021A1515010199/Natural Science Foundation of Guangdong Province
- 2021A1515010199/Natural Science Foundation of Guangdong Province
- 2021A1515010199/Natural Science Foundation of Guangdong Province
- 2020B111114002/Key Areas Research and Development Program of Guangdong
- 2020B111114002/Key Areas Research and Development Program of Guangdong
- 2020B111114002/Key Areas Research and Development Program of Guangdong
- 2020B111114002/Key Areas Research and Development Program of Guangdong
- 2020B111114002/Key Areas Research and Development Program of Guangdong
- 2020B111114002/Key Areas Research and Development Program of Guangdong
- 2020B111114002/Key Areas Research and Development Program of Guangdong
- 2020B111114002/Key Areas Research and Development Program of Guangdong
- 2020B111114002/Key Areas Research and Development Program of Guangdong
- 2020B1111170006/Guangdong Provincial Clinical Research Center for Urological Diseases
- 2020B1111170006/Guangdong Provincial Clinical Research Center for Urological Diseases
- 2020B1111170006/Guangdong Provincial Clinical Research Center for Urological Diseases
- 2020B1111170006/Guangdong Provincial Clinical Research Center for Urological Diseases
- 2020B1111170006/Guangdong Provincial Clinical Research Center for Urological Diseases
- 2020B1111170006/Guangdong Provincial Clinical Research Center for Urological Diseases
- 2020B1111170006/Guangdong Provincial Clinical Research Center for Urological Diseases
- 2020B1111170006/Guangdong Provincial Clinical Research Center for Urological Diseases
- 2020B1111170006/Guangdong Provincial Clinical Research Center for Urological Diseases
- 2020B1212060018/Guangdong Science and Technology Department
- 2020B1212060018/Guangdong Science and Technology Department
- 2020B1212060018/Guangdong Science and Technology Department
- 2020B1212060018/Guangdong Science and Technology Department
- 2020B1212060018/Guangdong Science and Technology Department
- 2020B1212060018/Guangdong Science and Technology Department
- 2020B1212060018/Guangdong Science and Technology Department
- 2020B1212060018/Guangdong Science and Technology Department
- 2020B1212060018/Guangdong Science and Technology Department
LinkOut - more resources
Full Text Sources
Medical

