Asymmetrical high-flow nasal cannula performs similarly to standard interface in patients with acute hypoxemic post-extubation respiratory failure: a pilot study
- PMID: 38191347
- PMCID: PMC10775427
- DOI: 10.1186/s12890-023-02820-x
Asymmetrical high-flow nasal cannula performs similarly to standard interface in patients with acute hypoxemic post-extubation respiratory failure: a pilot study
Abstract
Background: Standard high-flow nasal cannula (HFNC) is a respiratory support device widely used to manage post-extubation hypoxemic acute respiratory failure (hARF) due to greater comfort, oxygenation, alveolar recruitment, humidification, and reduction of dead space, as compared to conventional oxygen therapy. On the contrary, the effects of the new asymmetrical HFNC interface (Optiflow® Duet system (Fisher & Paykel, Healthcare, Auckland, New Zealand) is still under discussion. Our aim is investigating whether the use of asymmetrical HFNC interface presents any relevant difference, compared with the standard configuration, on lung aeration (as assessed by end-expiratory lung impedance (EELI) measured by electrical impedance tomography (EIT)), diaphragm ultrasound thickening fraction (TFdi) and excursion (DE), ventilatory efficiency (estimated by corrected minute ventilation (MV)), gas exchange, dyspnea, and comfort.
Methods: Pilot physiological crossover randomized controlled study enrolling 20 adults admitted to the Intensive Care unit, invasively ventilated for at least 24 h, and developing post-extubation hARF, i.e., PaO2/set FiO2 < 300 mmHg during Venturi mask (VM) within 120 min after extubation. Each HFNC configuration was applied in a randomized 60 min sequence at a flow rate of 60 L/min.
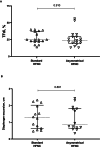
Results: Global EELI, TFdi, DE, ventilatory efficiency, gas exchange and dyspnea were not significantly different, while comfort was greater during asymmetrical HFNC support, as compared to standard interface (10 [7-10] and 8 [7-9], p-value 0.044).
Conclusions: In post-extubation hARF, the use of the asymmetrical HFNC, as compared to standard HFNC interface, slightly improved patient comfort without affecting lung aeration, diaphragm activity, ventilatory efficiency, dyspnea and gas exchange.
Clinical trial number: ClinicalTrial.gov.
Registration number: NCT05838326 (01/05/2023).
New & noteworthy: The asymmetrical high-flow nasal cannula oxygen therapy (Optiflow® Duet system (Fisher & Paykel, Healthcare, Auckland, New Zealand) provides greater comfort as compared to standard interface; while their performance in term of lung aeration, diaphragm activity, ventilatory efficiency, dyspnea, and gas exchange is similar.
Keywords: Asymmetrical cannula; DUET; High flow nasal oxygen; High flow nasal therapy; High-flow nasal cannula.
© 2024. The Author(s).
Conflict of interest statement
PN research lab received grants/research equipment by Draeger, Mindray, Intersurgical SPA and Gilead. PN receives royalties from Intersurgical SPA for Helmet Next invention. He also received speaking fees from Getinge, Intersurgical SPA, Gilead, MSD, Draeger. NS and FZ received speaking fees from Getinge. The other authors have no other competing interests to declare.
Figures

References
-
- Roca O, Hernández G, Díaz-Lobato S, Carratalá JM, Gutiérrez RM, Masclans JR, et al. Current evidence for the effectiveness of heated and humidified high flow nasal cannula supportive therapy in adult patients with Respiratory Failure. Crit Care Lond Engl. 2016;20(1):109. doi: 10.1186/s13054-016-1263-z. - DOI - PMC - PubMed

