Increased cysteinyl-tRNA synthetase drives neuroinflammation in Alzheimer's disease
- PMID: 38191451
- PMCID: PMC10773087
- DOI: 10.1186/s40035-023-00394-6
Increased cysteinyl-tRNA synthetase drives neuroinflammation in Alzheimer's disease
Abstract
Background: Microglia-mediated neuroinflammation in Alzheimer's disease (AD) is not only a response to pathophysiological events, but also plays a causative role in neurodegeneration. Cytoplasmic cysteinyl-tRNA synthetase (CARS) is considered to be a stimulant for immune responses to diseases; however, it remains unknown whether CARS is involved in the pathogenesis of AD.
Methods: Postmortem human temporal cortical tissues at different Braak stages and AD patient-derived serum samples were used to investigate the changes of CARS levels in AD by immunocytochemical staining, real-time PCR, western blotting and ELISA. After that, C57BL/6J and APP/PS1 transgenic mice and BV-2 cell line were used to explore the role of CARS protein in memory and neuroinflammation, as well as the underlying mechanisms. Finally, the associations of morphological features among CARS protein, microglia and dense-core plaques were examined by immunocytochemical staining.
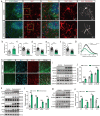
Results: A positive correlation was found between aging and the intensity of CARS immunoreactivity in the temporal cortex. Both protein and mRNA levels of CARS were increased in the temporal cortex of AD patients. Immunocytochemical staining revealed increased CARS immunoreactivity in neurons of the temporal cortex in AD patients. Moreover, overexpression of CARS in hippocampal neurons induced and aggravated cognitive dysfunction in C57BL/6J and APP/PS1 mice, respectively, accompanied by activation of microglia and the TLR2/MyD88 signaling pathway as well as upregulation of proinflammatory cytokines. In vitro experiments showed that CARS treatment facilitated the production of proinflammatory cytokines and the activation of the TLR2/MyD88 signaling pathway of BV-2 cells. The accumulation of CARS protein occurred within dense-core Aβ plaques accompanied by recruitment of ameboid microglia. Significant upregulation of TLR2/MyD88 proteins was also observed in the temporal cortex of AD.
Conclusions: The findings suggest that the neuronal CARS drives neuroinflammation and induces memory deficits, which might be involved in the pathogenesis of AD.
Keywords: Alzheimer’s disease; Cysteinyl-tRNA synthetase; Microglia; Neuroinflammation; TLR2.
© 2024. The Author(s).
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures





References
-
- Velandia PP, Miller-Petrie MK, Chen C, Chakrabarti S, Chapin A, Hay S, et al. Global and regional spending on dementia care from 2000–2019 and expected future health spending scenarios from 2020–2050: An economic modelling exercise. EClinicalMedicine. 2022;45:101337. doi: 10.1016/j.eclinm.2022.101337. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
- 32030046/the National Natural Science Foundation of China
- XDB32020200/the Strategic Priority Research Program of Chinese Academy of Science
- 2016YFC1305900/the National Key R&D Program of China
- KJ2021A0212/Anhui Province University Scientific Research Project
- 32000716/the National Natural Science Foundation of China
LinkOut - more resources
Full Text Sources
Medical

