Understanding the molecular mechanism of pathogenic variants of BIR2 domain in XIAP-deficient inflammatory bowel disease
- PMID: 38191507
- PMCID: PMC10774423
- DOI: 10.1038/s41598-023-50932-5
Understanding the molecular mechanism of pathogenic variants of BIR2 domain in XIAP-deficient inflammatory bowel disease
Abstract
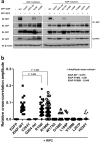
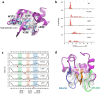
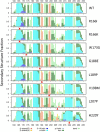
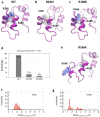
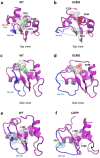
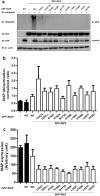
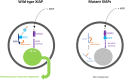
X-linked inhibitor of apoptosis protein (XIAP) deficiency causes refractory inflammatory bowel disease. The XIAP protein plays a pivotal role in the pro-inflammatory response through the nucleotide-binding oligomerization domain-containing signaling pathway that is important in mucosal homeostasis. We analyzed the molecular mechanism of non-synonymous pathogenic variants (PVs) of XIAP BIR2 domain. We generated N-terminally green fluorescent protein-tagged XIAP constructs of representative non-synonymous PVs. Co-immunoprecipitation and fluorescence cross-correlation spectroscopy showed that wild-type XIAP and RIP2 preferentially interacted in live cells, whereas all non-synonymous PV XIAPs failed to interact properly with RIP2. Structural analysis showed that various structural changes by mutations, such as hydrophobic core collapse, Zn-finger loss, and spatial rearrangement, destabilized the two loop structures (174-182 and 205-215) that critically interact with RIP2. Subsequently, it caused a failure of RIP2 ubiquitination and loss of protein deficiency by the auto-ubiquitination of all XIAP mutants. These findings could enhance our understanding of the role of XIAP mutations in XIAP-deficient inflammatory bowel disease and may benefit future therapeutic strategies.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures
Similar articles
-
Nucleotide-binding oligomerization domain (NOD) signaling defects and cell death susceptibility cannot be uncoupled in X-linked inhibitor of apoptosis (XIAP)-driven inflammatory disease.J Biol Chem. 2017 Jun 9;292(23):9666-9679. doi: 10.1074/jbc.M117.781500. Epub 2017 Apr 12. J Biol Chem. 2017. PMID: 28404814 Free PMC article.
-
Disease-causing mutations in the XIAP BIR2 domain impair NOD2-dependent immune signalling.EMBO Mol Med. 2013 Aug;5(8):1278-95. doi: 10.1002/emmm.201303090. Epub 2013 Jul 1. EMBO Mol Med. 2013. PMID: 23818254 Free PMC article.
-
Disruption of XIAP-RIP2 Association Blocks NOD2-Mediated Inflammatory Signaling.Mol Cell. 2018 Feb 15;69(4):551-565.e7. doi: 10.1016/j.molcel.2018.01.016. Mol Cell. 2018. PMID: 29452636
-
How genetic testing can lead to targeted management of XIAP deficiency-related inflammatory bowel disease.Genet Med. 2017 Feb;19(2):133-143. doi: 10.1038/gim.2016.82. Epub 2016 Jul 14. Genet Med. 2017. PMID: 27416006 Review.
-
X-linked inhibitor of apoptosis protein deficiency: more than an X-linked lymphoproliferative syndrome.J Clin Immunol. 2015 May;35(4):331-8. doi: 10.1007/s10875-015-0141-9. Epub 2015 Mar 4. J Clin Immunol. 2015. PMID: 25737324 Review.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

