O-GlcNAcylation mediates H2O2-induced apoptosis through regulation of STAT3 and FOXO1
- PMID: 38191912
- PMCID: PMC10943090
- DOI: 10.1038/s41401-023-01218-z
O-GlcNAcylation mediates H2O2-induced apoptosis through regulation of STAT3 and FOXO1
Abstract
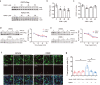
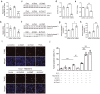
The O-linked-β-N-acetylglucosamine (O-GlcNAc) glycosylation (O-GlcNAcylation) is a critical post-translational modification that couples the external stimuli to intracellular signal transduction networks. However, the critical protein targets of O-GlcNAcylation in oxidative stress-induced apoptosis remain to be elucidated. Here, we show that treatment with H2O2 inhibited O-GlcNAcylation, impaired cell viability, increased the cleaved caspase 3 and accelerated apoptosis of neuroblastoma N2a cells. The O-GlcNAc transferase (OGT) inhibitor OSMI-1 or the O-GlcNAcase (OGA) inhibitor Thiamet-G enhanced or inhibited H2O2-induced apoptosis, respectively. The total and phosphorylated protein levels, as well as the promoter activities of signal transducer and activator of transcription factor 3 (STAT3) and Forkhead box protein O 1 (FOXO1) were suppressed by OSMI-1. In contrast, overexpressing OGT or treating with Thiamet-G increased the total protein levels of STAT3 and FOXO1. Overexpression of STAT3 or FOXO1 abolished OSMI-1-induced apoptosis. Whereas the anti-apoptotic effect of OGT and Thiamet-G in H2O2-treated cells was abolished by either downregulating the expression or activity of endogenous STAT3 or FOXO1. These results suggest that STAT3 or FOXO1 are the potential targets of O-GlcNAcylation involved in the H2O2-induced apoptosis of N2a cells.
Keywords: FOXO1; O-GlcNAcylation; STAT3; apoptosis; oxidative stress.
© 2024. The Author(s), under exclusive licence to Shanghai Institute of Materia Medica, Chinese Academy of Sciences and Chinese Pharmacological Society.
Conflict of interest statement
The authors declare no competing interests.
Figures







References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

