Ketone ester supplementation of Atkins-type diet prolongs survival in an orthotopic xenograft model of glioblastoma
- PMID: 38192123
- PMCID: PMC10968192
- DOI: 10.5115/acb.23.158
Ketone ester supplementation of Atkins-type diet prolongs survival in an orthotopic xenograft model of glioblastoma
Abstract
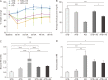
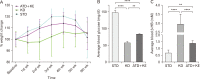
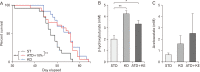
Heavy reliance on glucose metabolism and a reduced capacity to use ketone bodies makes glioblastoma (GBM) a promising candidate for ketone-based therapies. Ketogenic diet (KD) is well-known for its promising effects in controlling tumor growth in GBM. Moreover, synthetic ketone ester (KE) has demonstrated to increase blood ketone levels and enhance animal survival in a metastatic VM-M3 murine tumor model. Here, we compared the efficacy of a KE-supplemented Atkins-type diet (ATD-KE) to a classic KD in controlling tumor progression and enhancing survival in a clinically relevant orthotopic patient-derived xenograft GBM model. Our findings demonstrate that ATD-KE preserves body weight (percent change from the baseline; 112±2.99 vs. 116.9±2.52 and 104.8±3.67), decreases blood glucose (80.55±0.86 vs. 118.6±9.51 and 52.35±3.89 mg/dl), and increases ketone bodies in blood (1.15±0.03 mM vs. 0.55±0.04 and 2.66±0.21 mM) and brain tumor tissue (3.35±0.30 mM vs. 2.04±0.3 and 4.25±0.25 mM) comparable to the KD (results presented for ATD-KE vs. standard diet [STD] and KD, respectively). Importantly, the ATD-KE treatment significantly enhanced survival compared to the STD and was indistinguishable from the KD (47 days in STD vs. 56 days in KD and ATD-KE), suggesting that a nutritionally balanced low carbohydrate ATD combined with KE may be as effective as the KD alone in reducing brain tumor progression. Overall, these data support the rationale for clinical testing of KE-supplemented low-carb diet as an adjunct treatment for brain tumor patients.
Keywords: Glioblastoma; Ketone bodies; Ketone ester; Survival; Tumor progression.
Conflict of interest statement
No potential conflict of interest relevant to this article was reported.
Figures
Similar articles
-
Ketone supplementation decreases tumor cell viability and prolongs survival of mice with metastatic cancer.Int J Cancer. 2014 Oct 1;135(7):1711-20. doi: 10.1002/ijc.28809. Epub 2014 May 14. Int J Cancer. 2014. PMID: 24615175 Free PMC article.
-
Comparison of Exogenous Ketone Administration Versus Dietary Carbohydrate Restriction on Myocardial Glucose Suppression: A Crossover Clinical Trial.J Nucl Med. 2022 May;63(5):770-776. doi: 10.2967/jnumed.121.262734. Epub 2021 Oct 21. J Nucl Med. 2022. PMID: 34675108 Free PMC article. Clinical Trial.
-
Exogenous Ketones Lower Blood Glucose Level in Rested and Exercised Rodent Models.Nutrients. 2019 Oct 1;11(10):2330. doi: 10.3390/nu11102330. Nutrients. 2019. PMID: 31581549 Free PMC article.
-
Ketogenic Diet and Ketone Bodies as Clinical Support for the Treatment of SARS-CoV-2-Review of the Evidence.Viruses. 2023 May 27;15(6):1262. doi: 10.3390/v15061262. Viruses. 2023. PMID: 37376562 Free PMC article. Review.
-
On the nutritional and therapeutic effects of ketone body D-β-hydroxybutyrate.Appl Microbiol Biotechnol. 2021 Aug;105(16-17):6229-6243. doi: 10.1007/s00253-021-11482-w. Epub 2021 Aug 20. Appl Microbiol Biotechnol. 2021. PMID: 34415393 Free PMC article. Review.
Cited by
-
Oral Administration of a Novel, Synthetic Ketogenic Compound Elevates Blood β-Hydroxybutyrate Levels in Mice in Both Fasted and Fed Conditions.Nutrients. 2024 Oct 18;16(20):3526. doi: 10.3390/nu16203526. Nutrients. 2024. PMID: 39458521 Free PMC article.
-
Spatial mapping of phosphatidylcholine sn-positional isomers using CID of divalent metal complexes in imaging mass spectrometry.Int J Mass Spectrom. 2025 Feb;508:117370. doi: 10.1016/j.ijms.2024.117370. Epub 2024 Nov 22. Int J Mass Spectrom. 2025. PMID: 40778296
References
-
- Hau P, Baumgart U, Pfeifer K, Bock A, Jauch T, Dietrich J, Fabel K, Grauer O, Wismeth C, Klinkhammer-Schalke M, Allgäuer M, Schuierer G, Koch H, Schlaier J, Ulrich W, Brawanski A, Bogdahn U, Steinbrecher A. Salvage therapy in patients with glioblastoma: is there any benefit? Cancer. 2003;98:2678–86. doi: 10.1002/cncr.11845. - DOI - PubMed
-
- Hoang-Minh LB, Siebzehnrubl FA, Yang C, Suzuki-Hatano S, Dajac K, Loche T, Andrews N, Schmoll Massari M, Patel J, Amin K, Vuong A, Jimenez-Pascual A, Kubilis P, Garrett TJ, Moneypenny C, Pacak CA, Huang J, Sayour EJ, Mitchell DA, Sarkisian MR, Reynolds BA, Deleyrolle LP. Infiltrative and drug-resistant slow-cycling cells support metabolic heterogeneity in glioblastoma. EMBO J. 2018;37:e98772. doi: 10.15252/embj.201798772. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
