A translational MRI approach to validate acute axonal damage detection as an early event in multiple sclerosis
- PMID: 38192199
- PMCID: PMC10776086
- DOI: 10.7554/eLife.79169
A translational MRI approach to validate acute axonal damage detection as an early event in multiple sclerosis
Abstract
Axonal degeneration is a central pathological feature of multiple sclerosis and is closely associated with irreversible clinical disability. Current noninvasive methods to detect axonal damage in vivo are limited in their specificity and clinical applicability, and by the lack of proper validation. We aimed to validate an MRI framework based on multicompartment modeling of the diffusion signal (AxCaliber) in rats in the presence of axonal pathology, achieved through injection of a neurotoxin damaging the neuronal terminal of axons. We then applied the same MRI protocol to map axonal integrity in the brain of multiple sclerosis relapsing-remitting patients and age-matched healthy controls. AxCaliber is sensitive to acute axonal damage in rats, as demonstrated by a significant increase in the mean axonal caliber along the targeted tract, which correlated with neurofilament staining. Electron microscopy confirmed that increased mean axonal diameter is associated with acute axonal pathology. In humans with multiple sclerosis, we uncovered a diffuse increase in mean axonal caliber in most areas of the normal-appearing white matter, preferentially affecting patients with short disease duration. Our results demonstrate that MRI-based axonal diameter mapping is a sensitive and specific imaging biomarker that links noninvasive imaging contrasts with the underlying biological substrate, uncovering generalized axonal damage in multiple sclerosis as an early event.
Keywords: MRI; axonal pathology; multiple sclerosis; neuroscience; rat.
© 2024, Cerdán Cerdá, Toschi et al.
Conflict of interest statement
AC, NT, CT, VB, EH, AM, JG, CM, SD No competing interests declared
Figures






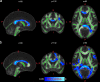
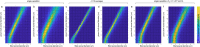
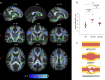
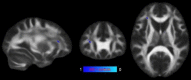


Update of
- doi: 10.1101/2022.04.27.489694
Similar articles
-
In vivo characterization of cortical and white matter neuroaxonal pathology in early multiple sclerosis.Brain. 2017 Nov 1;140(11):2912-2926. doi: 10.1093/brain/awx247. Brain. 2017. PMID: 29053798 Free PMC article.
-
Whole brain in vivo axonal diameter mapping in multiple sclerosis.Annu Int Conf IEEE Eng Med Biol Soc. 2019 Jul;2019:204-207. doi: 10.1109/EMBC.2019.8856433. Annu Int Conf IEEE Eng Med Biol Soc. 2019. PMID: 31945878
-
Myelin and axon pathology in multiple sclerosis assessed by myelin water and multi-shell diffusion imaging.Brain. 2021 Jul 28;144(6):1684-1696. doi: 10.1093/brain/awab088. Brain. 2021. PMID: 33693571 Free PMC article.
-
Imaging neuronal and axonal degeneration in multiple sclerosis.Neurol Sci. 2003 Dec;24 Suppl 5:S283-6. doi: 10.1007/s10072-003-0175-2. Neurol Sci. 2003. PMID: 14652790 Review.
-
The role of magnetic resonance techniques in understanding and managing multiple sclerosis.Brain. 1998 Jan;121 ( Pt 1):3-24. doi: 10.1093/brain/121.1.3. Brain. 1998. PMID: 9549485 Review.
Cited by
-
A focus on the normal-appearing white and gray matter within the multiple sclerosis brain: a link to smoldering progression.Acta Neuropathol. 2025 Aug 10;150(1):16. doi: 10.1007/s00401-025-02923-1. Acta Neuropathol. 2025. PMID: 40783892 Free PMC article. Review.
-
Simulation-Based Inference at the Theoretical Limit: Fast, Accurate Microstructural MRI with Minimal diffusion MRI Data.bioRxiv [Preprint]. 2025 Jul 28:2024.11.11.622925. doi: 10.1101/2024.11.11.622925. bioRxiv. 2025. PMID: 40766636 Free PMC article. Preprint.
References
-
- Barakovic M, Pizzolato M, Tax CMW, Rudrapatna U, Magon S, Dyrby TB, Granziera C, Thiran JP, Jones DK, Canales-Rodríguez EJ. Estimating axon radius using diffusion-relaxation MRI: calibrating a surface-based relaxation model with histology. Frontiers in Neuroscience. 2023;17:1209521. doi: 10.3389/fnins.2023.1209521. - DOI - PMC - PubMed
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

