A giant left atrial appendage: a case report on the feasibility of closure with a custom-made device
- PMID: 38192271
- PMCID: PMC10772948
- DOI: 10.1093/ehjcr/ytad629
A giant left atrial appendage: a case report on the feasibility of closure with a custom-made device
Abstract
Background: Transcatheter left atrial appendage occlusion (LAAO) is a valuable therapeutic option for stroke prevention in patients with atrial fibrillation (AF) at high bleeding risk. However, complex LAA anatomies sometimes preclude the adoption of commercially available LAAO devices. The design of a custom-made LAAO device is a promising strategy in these cases. However, few examples of custom-made devices in case of giant LAAs have been reported.
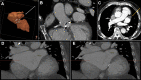
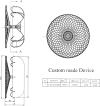
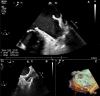
Case summary: An 85-year-old man with permanent AF with CHA2DS2-VASc 4 and recurrent active gastrointestinal major bleedings was referred for transcatheter LAAO at Parma University Hospital after multidisciplinary team evaluation. Pre-procedural coronary computed tomography angiography revealed a giant windsock LAA, with a maximum ostium diameter of 44 mm, a landing zone diameter of 34 mm, and maximal length of 49 mm. Patient's management was particularly challenging given that available LAAO devices were too small to completely exclude the LAA. In accordance with the manufacturer, a custom-made LAmbre™ Closure System (Lifetech Scientific, Shenzhen, China), which specifically fitted with patient's LAA anatomy, was designed and successfully deployed under transoesophageal echocardiography (TEE) and fluoroscopic guidance. Periprocedural TEE confirmed the appropriate position of the device and the absence of peri-device leaks. No adverse ischaemic and haemorrhagic events were reported at 3-months follow-up.
Discussion: We present a case of a successful transcatheter LAAO procedure by deploying a custom-made LAmbre device 38/46 mm to mechanically exclude a giant windsock LAA. This case illustrates the effectiveness of a custom-made device strategy, which potentially enables the closure of all complex LAA anatomies.
Keywords: Atrial fibrillation; Case report; Custom-made device; Giant left atrial appendage; LAmbre™ device; Left atrial appendage occlusion.
© The Author(s) 2024. Published by Oxford University Press on behalf of the European Society of Cardiology.
Conflict of interest statement
Conflict of interest: None declared.
Figures


References
-
- Hindricks G, Potpara T, Dagres N, Arbelo E, Bax JJ, Blomstrom-Lundqvist C, et al. 2020 ESC guidelines for the diagnosis and management of atrial fibrillation developed in collaboration with the European Association for Cardio-Thoracic Surgery (EACTS): the Task Force for the diagnosis and management of atrial fibrillation of the European Society of Cardiology (ESC) developed with the special contribution of the European Heart Rhythm Association (EHRA) of the ESC. Eur Heart J 2021;42:373–498. - PubMed
-
- Reddy VY, Doshi SK, Sievert H, Buchbinder M, Neuzil P, Huber K, et al. Percutaneous left atrial appendage closure for stroke prophylaxis in patients with atrial fibrillation: 2.3-year follow-up of the PROTECT AF (Watchman Left Atrial Appendage System for Embolic Protection in Patients with Atrial Fibrillation) trial. Circulation 2013;127:720–729. - PubMed
-
- Holmes HD Jr, Kar S, Price MJ, Whisenant B, Sievert H, Doshi SK, et al. Prospective randomized evaluation of the Watchman Left Atrial Appendage Closure device in patients with atrial fibrillation versus long-term warfarin therapy: the PREVAIL trial. J Am Coll Cardiol 2014;64:1–12. - PubMed
-
- Osmancik P, Herman D, Neuzil P, Hala P, Taborsky M, Kapa P, et al. Left atrial appendage closure versus direct oral anticoagulants in high-risk patients with atrial fibrillation. J Am Coll Cardiol 2020;75:3122–3135. - PubMed
-
- Tsai LM, Lin LJ, Teng JK, Chen JH. Prevalence and clinical significance of left atrial thrombus in nonrheumatic atrial fibrillation. Int J Cardiol 1997;58:163–169. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
