Evaluating the efficacy of purchased antisense oligonucleotides to reduce mouse and human tau in vivo
- PMID: 38192302
- PMCID: PMC10773814
- DOI: 10.3389/fnmol.2023.1320182
Evaluating the efficacy of purchased antisense oligonucleotides to reduce mouse and human tau in vivo
Abstract
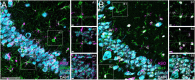
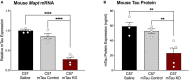
Many preclinical and clinical studies support the use of antisense oligonucleotides (ASOs) as effective therapeutic strategies. However, acquiring ASOs for research purposes may be limited by partnerships with the pharmaceutical companies. Our lab previously developed an effective ASO strategy to lower human tau and reverse pathology in aged tauopathy model mice. Testing the efficacy of purchased tau lowering ASOs would provide support for these reagents as broad research tools. Purchased mouse and human tau lowering ASOs were infused or injected intracerebroventricularly into wildtype and tau transgenic mice. Following treatment, brain tissue evaluated for ASO distribution and levels of tau mRNA, protein, and phosphorylated tau. We show that purchased ASOs enter cell types of the brain and effectively decrease mouse or human tau mRNA and protein levels. Human tau lowering ASO treatment in PS19 mice decreased phosphorylated tau and gliosis relative to saline-treated PS19 mice, consistent with our previous study using a non-commercial tau lowering ASO. The results of this study demonstrate the efficacy of purchased tau targeting ASOs in vivo to support their broad use by researchers.
Keywords: Alzheimer’s disease; antisense oligonucleotides; human tau mouse model; tau protein; tauopathies.
Copyright © 2023 Vemula, Schoch and Miller.
Conflict of interest statement
TM is a consultant for Ionis Pharmaceuticals and has a licensing agreement with Ionis regarding use of tau ASOs in neurodegenerative syndrome. TM is an inventor on patent/patent application PCT/US2013/031500, nationalized to US Issued Patent 10,273,474 (with corresponding national stage applications or issued patents in Australia, Canada, Europe, and Japan), and on continuation or divisional patent applications (US patent application number 16/298,607 and Australia Issued Patent 2016202220) regarding use of tau ASOs in neurodegenerative syndrome. TM has a licensing agreement with C2N Diagnostics, is a consultant for Biogen, Bioio, Cytokinetics, Disarm Therapeutics, and UCB, and received honorarium from Regeneron Pharmaceuticals and Denali Therapeutics. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


Similar articles
-
Tau reduction prevents neuronal loss and reverses pathological tau deposition and seeding in mice with tauopathy.Sci Transl Med. 2017 Jan 25;9(374):eaag0481. doi: 10.1126/scitranslmed.aag0481. Sci Transl Med. 2017. PMID: 28123067 Free PMC article.
-
Biologic TNF-α inhibitors reduce microgliosis, neuronal loss, and tau phosphorylation in a transgenic mouse model of tauopathy.J Neuroinflammation. 2021 Dec 31;18(1):312. doi: 10.1186/s12974-021-02332-7. J Neuroinflammation. 2021. PMID: 34972522 Free PMC article.
-
The development of peptide- and oligonucleotide-based drugs to prevent the formation of abnormal tau in tauopathies.Expert Opin Drug Discov. 2023 May;18(5):515-526. doi: 10.1080/17460441.2023.2200245. Epub 2023 Apr 11. Expert Opin Drug Discov. 2023. PMID: 37042028 Review.
-
Identification and characterization of a MAPT-targeting locked nucleic acid antisense oligonucleotide therapeutic for tauopathies.Mol Ther Nucleic Acids. 2022 Aug 4;29:625-642. doi: 10.1016/j.omtn.2022.07.027. eCollection 2022 Sep 13. Mol Ther Nucleic Acids. 2022. PMID: 36090761 Free PMC article.
-
Joining forces to develop individualized antisense oligonucleotides for patients with brain or eye diseases: the example of the Dutch Center for RNA Therapeutics.Ther Adv Rare Dis. 2024 Sep 23;5:26330040241273465. doi: 10.1177/26330040241273465. eCollection 2024 Jan-Dec. Ther Adv Rare Dis. 2024. PMID: 39328974 Free PMC article. Review.
Cited by
-
On the reversibility of amyloid fibril formation.Biophys Rev (Melville). 2025 Jan 29;6(1):011303. doi: 10.1063/5.0236947. eCollection 2025 Mar. Biophys Rev (Melville). 2025. PMID: 39973975 Free PMC article. Review.
References
-
- Biogen (2022). Biogen and Ionis Announce Topline Phase 1 Study Results of Investigational Drug in C9orf72 Amyotrophic Lateral Sclerosis. Munich: Biogen.
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

