Combined population genomic screening for three high-risk conditions in Australia: a modelling study
- PMID: 38192593
- PMCID: PMC10772163
- DOI: 10.1016/j.eclinm.2023.102297
Combined population genomic screening for three high-risk conditions in Australia: a modelling study
Abstract
Background: No previous health-economic evaluation has assessed the impact and cost-effectiveness of offering combined adult population genomic screening for mutliple high-risk conditions in a national public healthcare system.
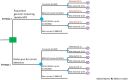
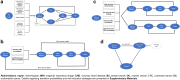
Methods: This modeling study assessed the impact of offering combined genomic screening for hereditary breast and ovarian cancer, Lynch syndrome and familial hypercholesterolaemia to all young adults in Australia, compared with the current practice of clinical criteria-based testing for each condition separately. The intervention of genomic screening, assumed as an up-front single cost in the first annual model cycle, would detect pathogenic variants in seven high-risk genes. The simulated population was 18-40 year-olds (8,324,242 individuals), modelling per-sample test costs ranging AU$100-$1200 (base-case AU$200) from the year 2023 onwards with testing uptake of 50%. Interventions for identified high-risk variant carriers follow current Australian guidelines, modelling imperfect uptake and adherence. Outcome measures were morbidity and mortality due to cancer (breast, ovarian, colorectal and endometrial) and coronary heart disease (CHD) over a lifetime horizon, from healthcare-system and societal perspectives. Outcomes included quality-adjusted life years (QALYs) and incremental cost-effectiveness ratio (ICER), discounted 5% annually (with 3% discounting in scenario analysis).
Findings: Over the population lifetime (to age 80 years), the model estimated that genomic screening per-100,000 individuals would lead to 747 QALYs gained by preventing 63 cancers, 31 CHD cases and 97 deaths. In the total model population, this would translate to 31,094 QALYs gained by preventing 2612 cancers, 542 non-fatal CHD events and 4047 total deaths. At AU$200 per-test, genomic screening would require an investment of AU$832 million for screening of 50% of the population. Our findings suggest that this intervention would be cost-effective from a healthcare-system perspective, yielding an ICER of AU$23,926 (∼£12,050/€14,110/US$15,345) per QALY gained over the status quo. In scenario analysis with 3% discounting, an ICER of AU$4758/QALY was obtained. Sensitivity analysis for the base case indicated that combined genomic screening would be cost-effective under 70% of simulations, cost-saving under 25% and not cost-effective under 5%. Threshold analysis showed that genomic screening would be cost-effective under the AU$50,000/QALY willingness-to-pay threshold at per-test costs up to AU$325 (∼£164/€192/US$208).
Interpretation: Our findings suggest that offering combined genomic screening for high-risk conditions to young adults would be cost-effective in the Australian public healthcare system, at currently realistic testing costs. Other matters, including psychosocial impacts, ethical and societal issues, and implementation challenges, also need consideration.
Funding: Australian Government, Department of Health, Medical Research Future Fund, Genomics Health Futures Mission (APP2009024). National Heart Foundation Future Leader Fellowship (102604).
Keywords: Cost-effectiveness analysis; Genomic testing; Genomics; Health-economic evaluation; Population screening; Prevention; Public health.
© 2023 The Author(s).
Conflict of interest statement
RM. declares advisory board membership from Astrazeneca/MSD/EGL/GSK. RCG. has received compensation for advising Allelica, Fabric, GenomeWeb, Genomic Life and Verily; and is a co-founder of Genome Medical and Nurture Genomics. KC. is co-Principal Investigator (PI) of an investigator-initiated trial of cervical screening, Compass, run by the Australian Centre for Prevention of Cervical Cancer (ACPCC), which is a government-funded not-for-profit charity; the ACPCC has received equipment and a funding contribution from Roche Molecular Diagnostics, and operational support from the Australian Government. KC. is also co-PI on a major investigator-initiated implementation program Elimination of Cervical Cancer in the Western Pacific (ECCWP) receives support from the Minderoo Foundation and equipment donations from Cepheid Inc. No other authors declare competing interests.
Figures


References
-
- Khoury M.J., McCabe L.L., McCabe E.R. Population screening in the age of genomic medicine. N Engl J Med. 2003;348(1):50–58. - PubMed
-
- Murray M.F., Evans J.P., Khoury M.J. DNA-based population screening: potential suitability and important knowledge gaps. JAMA. 2020;323(4):307–308. - PubMed
-
- Murray M.F., Giovanni M.A., Doyle D.L., et al. DNA-based screening and population health: a points to consider statement for programs and sponsoring organizations from the American College of Medical Genetics and Genomics (ACMG) Genet Med. 2021;23(6):989–995. - PubMed
-
- Grzymski J.J., Elhanan G., Morales Rosado J.A., et al. Population genetic screening efficiently identifies carriers of autosomal dominant diseases. Nat Med. 2020;26(8):1235–1239. - PubMed
LinkOut - more resources
Full Text Sources

