Chemotaxonomy, antibacterial and antioxidant activities of selected aromatic plants from Tabuk region-KSA
- PMID: 38192876
- PMCID: PMC10772130
- DOI: 10.1016/j.heliyon.2023.e23641
Chemotaxonomy, antibacterial and antioxidant activities of selected aromatic plants from Tabuk region-KSA
Abstract
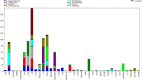
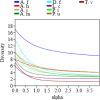
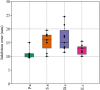
Chemotaxonomy is a valuable tool for obtaining taxonomic insights, which are most effectively employed in combination with other forms of data to establish a system of classification that closely reflects natural connections. The utilization of plant secondary metabolites possessing diverse therapeutic qualities signifies the growing exploitation of natural products in the medical discipline. The objectives of the current study encompassed the identification of phytochemicals in the extracts of nine species of medicinal plants, the examination of their chemotaxonomic properties, and the assessment of the antibacterial and antioxidant capabilities exhibited by the extracts. GC-MS technology was employed for the identification of phytochemical compounds. The study utilized ClassyFire, an automated chemical classification system that incorporates an extensive and computable classification, to categorize chemicals. The chemical classification of plants was examined by the application of principal component analysis (PCA) and cluster analysis (CA). The bactericidal properties of plants were assessed against four harmful bacterial species using the disc diffusion technique. The antioxidant properties of plant extracts were assessed employing the DPPH free radical scavenging methodology, and the half maximal effective concentration (EC50) was determined using dose response models. The calculator being referred to is the Quest Graph™ EC50 Calculator. In the plant extracts, the analysis disclosed the occurrence of 160 phytochemicals, classified into 36 phytochemical classes. The results of CA and PCA demonstrated the proximity and associations among Asteraceae species, while indicating the divergence of the two Lamiaceae species. Achillea fragrantissima and Ducrosia flabellifolia demonstrated the most diversity in phytochemical classes, while Thymus vulgaris displayed the highest level of dominance. Pulicaria undulata and T. vulgaris had the most notable antibacterial activity. D. flabellifolia and P. incisa demonstrated the highest levels of antioxidant activity. Ethanol exhibited superior antibacterial efficacy compared to other solvents. The remarkable biological activities exhibited by these plant extracts can be ascribed to the copious presence of certain chemicals, predominantly sesquiterpenoids, monoterpenoids, benzene and its derivatives, naphthalenes, fatty acyls, and phenols. The susceptibility of Gram-positive bacterial species to plant extracts was shown to be higher in comparison to Gram-negative bacterial species.
Keywords: Antibacterial; Antioxidant activity; Chemotaxonomy; Medicinal plants.
© 2023 Published by Elsevier Ltd.
Conflict of interest statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures






References
-
- Ayoub Z., Archana M., Siddhartha K., Mishra L. J. Bot. Soc. 2017;48
-
- Pirmoradian M., Hooshmand T. In: In Woodhead Publishing Series in Biomaterials, Applications of Nanocomposite Materials in Dentistry. Abdullah M., Asiri M., Inamuddin, Ali Mohammad, editors. Woodhead Publishing; 2019. Remineralization and antibacterial capabilities of resin-based dental nanocomposites; pp. 237–269.
-
- Bhatt I., Rawat S., Rawal R. 2013. Antioxidants in Medicinal Plants. - DOI
LinkOut - more resources
Full Text Sources
Miscellaneous

