FcγRIIb Exacerbates LPS-Induced Neuroinflammation by Binding with the Bridging Protein DAP12 and Promoting the Activation of PI3K/AKT Signaling Pathway in Microglia
- PMID: 38193040
- PMCID: PMC10773454
- DOI: 10.2147/JIR.S428093
FcγRIIb Exacerbates LPS-Induced Neuroinflammation by Binding with the Bridging Protein DAP12 and Promoting the Activation of PI3K/AKT Signaling Pathway in Microglia
Abstract
Introduction: This paper focuses on the expression and role of FcγRIIb in neuroinflammation, exploring the molecular mechanisms by which FcγRIIb interacts with the bridging protein DAP12 to regulate the PI3K-AKT signaling pathway that promote neuroinflammation and aggravate neuronal injury.
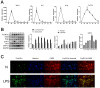
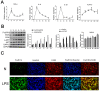
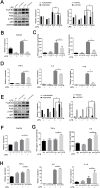
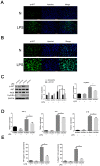
Methods: LPS-induced neuroinflammation models in vivo and in vitro were constructed to explore the role and mechanism of FcγRIIb in CNS inflammation. Subsequently, FcγRIIb was knocked down or overexpressed to observe the activation of BV2 cell and the effect on PI3K-AKT pathway. Then the PI3K-AKT pathway was blocked to observe its effect on cell activation and FcγRIIb expression. We analyzed the interaction between FcγRIIb and DAP12 by Immunoprecipitation technique. Then FcγRIIb was overexpressed while knocking down DAP12 to observe its effect on PI3K-AKT pathway. Finally, BV2 cell culture supernatant was co-cultured with neuronal cell HT22 to observe its effect on neuronal apoptosis and cell activity.
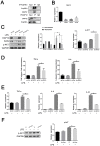
Results: In vivo and in vitro, we found that FcγRIIb expression was significantly increased and activated the PI3K-AKT pathway. Contrary to the results of overexpression of FcγRIIb, knockdown of FcγRIIb resulted in a significant low level of relevant inflammatory factors and suppressed the PI3K-AKT pathway. Furthermore, LPS stimulation induced an interaction between FcγRIIb and DAP12. Knockdown of DAP12 suppressed inflammation and activation of the PI3K-AKT pathway in BV2 cells, and meantime overexpression of FcγRIIb suppressed the level of FcγRIIb-induced AKT phosphorylation. Additionally, knockdown of FcγRIIb inhibited microglia activation, which induced neuronal apoptosis.
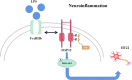
Discussion: Altogether, our experiments indicate that FcγRIIb interacts with DAP12 to promote microglia activation by activating the PI3K-AKT pathway while leading to neuronal apoptosis and exacerbating brain tissue injury, which may provide a new target for the treatment of inflammatory diseases in the central nervous system.
Keywords: DAP12; FcγRIIb; LPS-induced neuroinflammation; PI3K-AKT; microglia.
© 2024 Han et al.
Conflict of interest statement
Prof. Dr. Xiaoyi Shao reports grants from Jiangsu Education Department, during the conduct of the study. The authors report no other conflicts of interest in this work.
Figures

Similar articles
-
Tripartite-motif protein 21 knockdown extenuates LPS-triggered neurotoxicity by inhibiting microglial M1 polarization via suppressing NF-κB-mediated NLRP3 inflammasome activation.Arch Biochem Biophys. 2021 Jul 30;706:108918. doi: 10.1016/j.abb.2021.108918. Epub 2021 May 13. Arch Biochem Biophys. 2021. PMID: 33992596
-
TIPE2 regulates the response of BV2 cells to lipopolysaccharide by the crosstalk between PI3K/AKT signaling and microglia M1/M2 polarization.Int Immunopharmacol. 2023 Jul;120:110389. doi: 10.1016/j.intimp.2023.110389. Epub 2023 May 26. Int Immunopharmacol. 2023. PMID: 37245300
-
Achyranthes bidentata polypeptide alleviates neurotoxicity of lipopolysaccharide-activated microglia via PI3K/Akt dependent NOX2/ROS pathway.Ann Transl Med. 2021 Oct;9(20):1522. doi: 10.21037/atm-21-4027. Ann Transl Med. 2021. PMID: 34790728 Free PMC article.
-
Isobutyrylshikonin inhibits lipopolysaccharide-induced nitric oxide and prostaglandin E2 production in BV2 microglial cells by suppressing the PI3K/Akt-mediated nuclear transcription factor-κB pathway.Nutr Res. 2014 Dec;34(12):1111-9. doi: 10.1016/j.nutres.2014.10.002. Epub 2014 Oct 7. Nutr Res. 2014. PMID: 25454762
-
Microglial TREM2/DAP12 Signaling: A Double-Edged Sword in Neural Diseases.Front Cell Neurosci. 2018 Aug 6;12:206. doi: 10.3389/fncel.2018.00206. eCollection 2018. Front Cell Neurosci. 2018. PMID: 30127720 Free PMC article. Review.
References
LinkOut - more resources
Full Text Sources

