Breakthrough infections after COVID-19 vaccinations do not elicit platelet hyperactivation and are associated with high platelet-lymphocyte and low platelet-neutrophil aggregates
- PMID: 38193050
- PMCID: PMC10772876
- DOI: 10.1016/j.rpth.2023.102262
Breakthrough infections after COVID-19 vaccinations do not elicit platelet hyperactivation and are associated with high platelet-lymphocyte and low platelet-neutrophil aggregates
Abstract
Background: Severe COVID-19 is associated with an excessive immunothrombotic response and thromboinflammatory complications. Vaccinations effectively reduce the risk of severe clinical outcomes in patients with COVID-19, but their impact on platelet activation and immunothrombosis during breakthrough infections is not known.
Objectives: To investigate how preemptive vaccinations modify the platelet-immune crosstalk during COVID-19 infections.
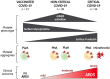
Methods: Cross-sectional flow cytometry study of the phenotype and interactions of platelets circulating in vaccinated (n = 21) and unvaccinated patients with COVID-19, either admitted to the intensive care unit (ICU, n = 36) or not (non-ICU, n = 38), in comparison to matched SARS-CoV-2-negative patients (n = 48), was performed.
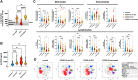
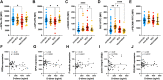
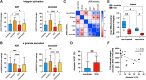
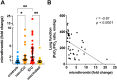
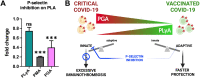
Results: In the circulation of unvaccinated non-ICU patients with COVID-19, we detected hyperactive and hyperresponsive platelets and platelet aggregates with adaptive and innate immune cells. In unvaccinated ICU patients with COVID-19, most of whom had severe acute respiratory distress syndrome, platelets had high P-selectin and phosphatidylserine exposure but low capacity to activate integrin αIIbβ3, dysfunctional mitochondria, and reduced surface glycoproteins. In addition, in the circulation of ICU patients, we detected microthrombi and platelet aggregates with innate, but not with adaptive, immune cells. In vaccinated patients with COVID-19, who had no acute respiratory distress syndrome, platelets had surface receptor levels comparable to those in controls and did not form microthrombi or platelet-granulocyte aggregates but aggregated avidly with adaptive immune cells.
Conclusion: Our study provides evidence that vaccinated patients with COVID-19 are not associated with platelet hyperactivation and are characterized by platelet-leukocyte aggregates that foster immune protection but not excessive immunothrombosis. These findings advocate for the importance of vaccination in preventing severe COVID-19.
Keywords: COVID-19; immunothrombosis; platelet aggregates; respiratory distress syndrome; vaccination.
© 2023 The Author(s).
Figures

Similar articles
-
Immunothrombotic Dysregulation in COVID-19 Pneumonia Is Associated With Respiratory Failure and Coagulopathy.Circulation. 2020 Sep 22;142(12):1176-1189. doi: 10.1161/CIRCULATIONAHA.120.048488. Epub 2020 Jul 28. Circulation. 2020. PMID: 32755393 Free PMC article.
-
Platelets' morphology, metabolic profile, exocytosis, and heterotypic aggregation with leukocytes in relation to severity and mortality of COVID-19-patients.Front Immunol. 2022 Nov 21;13:1022401. doi: 10.3389/fimmu.2022.1022401. eCollection 2022. Front Immunol. 2022. PMID: 36479107 Free PMC article.
-
Disease Severity in Moderate-to-Severe COVID-19 Is Associated With Platelet Hyperreactivity and Innate Immune Activation.Front Immunol. 2022 Mar 11;13:844701. doi: 10.3389/fimmu.2022.844701. eCollection 2022. Front Immunol. 2022. PMID: 35359931 Free PMC article.
-
Platelets and COVID-19.Hamostaseologie. 2021 Oct;41(5):379-385. doi: 10.1055/a-1581-4355. Epub 2021 Oct 25. Hamostaseologie. 2021. PMID: 34695854 Review.
-
Activated Platelets and Platelet-Derived Extracellular Vesicles Mediate COVID-19-Associated Immunothrombosis.Front Cell Dev Biol. 2022 Jul 6;10:914891. doi: 10.3389/fcell.2022.914891. eCollection 2022. Front Cell Dev Biol. 2022. PMID: 35874830 Free PMC article. Review.
References
LinkOut - more resources
Full Text Sources
Miscellaneous

