P2X7-Mediated Antigen-Independent Activation of CD8+ T Cells Promotes Salt-Sensitive Hypertension
- PMID: 38193292
- PMCID: PMC10922507
- DOI: 10.1161/HYPERTENSIONAHA.123.21819
P2X7-Mediated Antigen-Independent Activation of CD8+ T Cells Promotes Salt-Sensitive Hypertension
Abstract
Background: CD8+ T cells (CD8Ts) have been implicated in hypertension. However, the specific mechanisms are not fully understood. In this study, we explore the contribution of the P2X7 (purinergic receptor P2X7) receptor to CD8T activation and subsequent promotion of sodium retention in the kidney.
Methods: We used mouse models of hypertension. Wild type were used as genetic controls, OT1 and Rag2/OT1 mice were utilized to determine antigen dependency, and P2X7-knockout mice were studied to define the role of P2X7 in activating CD8Ts and promoting hypertension. Blood pressure was monitored continuously and kidneys were obtained at different experimental end points. Freshly isolated CD8Ts from mice for activation assays and ATP stimulation. CD8T activation-induced promotion of sodium retention was explored in cocultures of CD8Ts and mouse DCTs.
Results: We found that OT1 and Rag2/OT1 mice, which are nonresponsive to common antigens, still developed hypertension and CD8T-activation in response to deoxycorticosterone acetate/salt treatment, similar to wild-type mice. Further studies identified the P2X7 receptor on CD8Ts as a possible mediator of this antigen-independent activation of CD8Ts in hypertension. Knockout of the P2X7 receptor prevented calcium influx and cytokine production in CD8Ts. This finding was associated with reduced CD8T-DCT stimulation, reversal of excessive salt retention in DCTs, and attenuated development of salt-sensitive hypertension.
Conclusions: Our findings suggest a novel mechanism by which CD8Ts are activated in hypertension to exacerbate salt retention and infer that the P2X7 receptor on CD8Ts may represent a new therapeutic target to attenuate T-cell-mediated immunopathology in hypertension.
Keywords: blood pressure; cytokines; hypertension; kidney; sodium chloride.
Conflict of interest statement
Figures
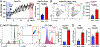
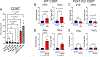



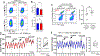
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

