Real-world treatment outcomes of immune checkpoint inhibitors used off-label in oncology: A comprehensive cancer institution experience
- PMID: 38193611
- PMCID: PMC10775181
- DOI: 10.1002/prp2.1167
Real-world treatment outcomes of immune checkpoint inhibitors used off-label in oncology: A comprehensive cancer institution experience
Abstract
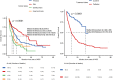
Off-label use (OLU) is quite common in oncology due to the complexity of cancer and the time-consuming regulatory process. However, outcomes of OLU in cancer treatment remain unclear. This study aimed to evaluate the overall survival (OS), event-free survival (EFS), duration of treatment (DOT), and reason for treatment discontinuation in patients receiving immune checkpoint inhibitors (ICI) as OLU for solid tumors from 2011 to 2020. The study collected data on 356 episodes (353 patients), with a median age of 64.4 years, 36.2% women, and 14.6% ECOG ≥ 2. Median OS was 15.7 (11.9-18.7) months, and median EFS was 5.4 (3.8-6.6) months. Men, patients with metastatic disease or ECOG-PS higher than 1, had worse survival outcomes. The findings derived from this study provide valuable information regarding the real-world use of ICI-OLU and contributes to enhancing the decision-making process for individuals with cancer. Further research on immunotherapy outcomes of OLU in cancer is needed.
Keywords: cancer; immune checkpoint inhibitors; off-label use; survival; treatment outcomes.
© 2024 The Authors. Pharmacology Research & Perspectives published by British Pharmacological Society and American Society for Pharmacology and Experimental Therapeutics and John Wiley & Sons Ltd.
Conflict of interest statement
The author(s) declare(s) that there are no conflicts of interest regarding the publication of this article.
Figures
References
-
- Weda M, Hoebert J, Moltó Puigmarti C, et al. Study on off‐label use of medicinal products in the European Union. Eur Union. 2017;1‐185. https://op.europa.eu/en/publication‐detail/‐/publication/ecf85518‐d376‐1...
-
- Drogovoz SM, Starikov VI, Ivantsyk LB, Shchokina KG. Experience and prospects for the use of off‐label drugs in oncology. Exp Oncol [Internet]. 2021;43(1):1‐5. https://pubmed.ncbi.nlm.nih.gov/33785726/ - PubMed
-
- Saiyed MM, Ong PS, Chew L. Off‐label drug use in oncology: a systematic review of literature. J Clin Pharm Ther. 2017;42(3):251‐258. - PubMed
-
- Levêque D. Off‐label use of targeted therapies in oncology. World J Clin Oncol [Internet]. 2016;7(2):253‐257. http://www.ncbi.nlm.nih.gov/pubmed/27081648 - PMC - PubMed
-
- From laboratory to patient: the journey of a medicine assessed by EMA [Internet]. [cited 2021 Feb 10]. https://www.ema.europa.eu/en/documents/other/laboratory‐patient‐journey‐...