Mutational profiling of mitochondrial DNA reveals an epithelial ovarian cancer-specific evolutionary pattern contributing to high oxidative metabolism
- PMID: 38193640
- PMCID: PMC10775184
- DOI: 10.1002/ctm2.1523
Mutational profiling of mitochondrial DNA reveals an epithelial ovarian cancer-specific evolutionary pattern contributing to high oxidative metabolism
Abstract
Background: Epithelial ovarian cancer (EOC) heavily relies on oxidative phosphorylation (OXPHOS) and exhibits distinct mitochondrial metabolic reprogramming. Up to now, the evolutionary pattern of somatic mitochondrial DNA (mtDNA) mutations in EOC tissues and their potential roles in metabolic remodelling have not been systematically elucidated.
Methods: Based on a large somatic mtDNA mutation dataset from private and public EOC cohorts (239 and 118 patients, respectively), we most comprehensively characterised the EOC-specific evolutionary pattern of mtDNA mutations and investigated its biological implication.
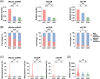
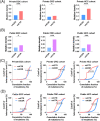
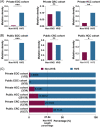
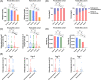
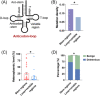
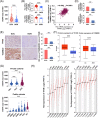
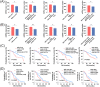
Results: Mutational profiling revealed that the mitochondrial genome of EOC tissues was highly unstable compared with non-cancerous ovary tissues. Furthermore, our data indicated the delayed heteroplasmy accumulation of mtDNA control region (mtCTR) mutations and near-complete absence of mtCTR non-hypervariable segment (non-HVS) mutations in EOC tissues, which is consistent with stringent negative selection against mtCTR mutation. Additionally, we observed a bidirectional and region-specific evolutionary pattern of mtDNA coding region mutations, manifested as significant negative selection against mutations in complex V (ATP6/ATP8) and tRNA loop regions, and potential positive selection on mutations in complex III (MT-CYB). Meanwhile, EOC tissues showed higher mitochondrial biogenesis compared with non-cancerous ovary tissues. Further analysis revealed the significant association between mtDNA mutations and both mitochondrial biogenesis and overall survival of EOC patients.
Conclusions: Our study presents a comprehensive delineation of EOC-specific evolutionary patterns of mtDNA mutations that aligned well with the specific mitochondrial metabolic remodelling, conferring novel insights into the functional roles of mtDNA mutations in EOC tumourigenesis and progression.
Keywords: epithelial ovarian cancer; evolutionary selection; metabolic remodelling; mitochondrial DNA; somatic mutations.
© 2024 The Authors. Clinical and Translational Medicine published by John Wiley & Sons Australia, Ltd on behalf of Shanghai Institute of Clinical Bioinformatics.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures
Similar articles
-
Mutational signature of mtDNA confers mechanistic insight into oxidative metabolism remodeling in colorectal cancer.Theranostics. 2023 Jan 1;13(1):324-338. doi: 10.7150/thno.78718. eCollection 2023. Theranostics. 2023. PMID: 36593960 Free PMC article.
-
Mutational profiling of mtDNA control region reveals tumor-specific evolutionary selection involved in mitochondrial dysfunction.EBioMedicine. 2022 Jun;80:104058. doi: 10.1016/j.ebiom.2022.104058. Epub 2022 May 17. EBioMedicine. 2022. PMID: 35594659 Free PMC article.
-
NGS-based profiling reveals a critical contributing role of somatic D-loop mtDNA mutations in HBV-related hepatocarcinogenesis.Ann Oncol. 2019 Jun 1;30(6):953-962. doi: 10.1093/annonc/mdz105. Ann Oncol. 2019. PMID: 30887045
-
Mitochondrial DNA mutations and oxidative damage in aging and diseases: an emerging paradigm of gerontology and medicine.Proc Natl Sci Counc Repub China B. 1998 Apr;22(2):55-67. Proc Natl Sci Counc Repub China B. 1998. PMID: 9615468 Review.
-
Somatic mitochondrial DNA mutations in human cancers.Adv Clin Chem. 2012;57:99-138. doi: 10.1016/b978-0-12-394384-2.00004-8. Adv Clin Chem. 2012. PMID: 22870588 Review.
Cited by
-
Exploring Strategies to Prevent and Treat Ovarian Cancer in Terms of Oxidative Stress and Antioxidants.Antioxidants (Basel). 2025 Jan 20;14(1):114. doi: 10.3390/antiox14010114. Antioxidants (Basel). 2025. PMID: 39857448 Free PMC article. Review.
-
Exosomal ncRNAs in reproductive cancers†.Biol Reprod. 2025 Feb 14;112(2):225-244. doi: 10.1093/biolre/ioae170. Biol Reprod. 2025. PMID: 39561105 Free PMC article. Review.
-
Identification of Ovarian High-Grade Serous Carcinoma with Mitochondrial Gene Variation.Int J Mol Sci. 2025 Feb 5;26(3):1347. doi: 10.3390/ijms26031347. Int J Mol Sci. 2025. PMID: 39941116 Free PMC article.
-
Unlocking the mitochondrial functional code: unraveling the pathogenesis of ovarian cancer and innovative targets to inhibit malignant behavior.J Transl Med. 2025 Jul 24;23(1):819. doi: 10.1186/s12967-025-06840-5. J Transl Med. 2025. PMID: 40707948 Free PMC article. Review.
-
Advances in predicting breast cancer driver mutations: Tools for precision oncology (Review).Int J Mol Med. 2025 Jan;55(1):6. doi: 10.3892/ijmm.2024.5447. Epub 2024 Oct 25. Int J Mol Med. 2025. PMID: 39450552 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
- 81830070/National Natural Science Foundation of China
- 82273376/National Natural Science Foundation of China
- 2023-ZDLSF-46/Key Research and Development Projects in Shaanxi Province
- CBSKL2022ZZ07/Autonomous Project of State Key Laboratory of Holistic Integrative Management of Gastrointestinal Cancers, China
LinkOut - more resources
Full Text Sources
Medical
