c-Jun signaling during initial HSV-1 infection modulates latency to enhance later reactivation in addition to directly promoting the progression to full reactivation
- PMID: 38193709
- PMCID: PMC10878265
- DOI: 10.1128/jvi.01764-23
c-Jun signaling during initial HSV-1 infection modulates latency to enhance later reactivation in addition to directly promoting the progression to full reactivation
Abstract
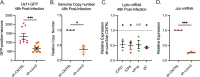
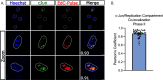
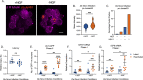
Herpes simplex virus-1 (HSV-1) establishes a latent infection in peripheral neurons and periodically reactivates to permit transmission, which can result in clinical manifestations. Viral transactivators required for lytic infection are largely absent during latent infection, and therefore, HSV-1 relies on the co-option of neuronal host signaling pathways to initiate its gene expression. The activation of the neuronal c-Jun N-terminal kinase (JNK) cell stress pathway is central to initiating biphasic reactivation in response to multiple stimuli. However, how host factors work with JNK to stimulate the initial wave of gene expression (known as Phase I) or the progression to full Phase II reactivation remains unclear. Here, we found that c-Jun, the primary target downstream of neuronal JNK cell stress signaling, functions during reactivation but not during the JNK-mediated initiation of Phase I gene expression. Instead, c-Jun was required to transition from Phase I to full HSV-1 reactivation and was detected in viral replication compartments of reactivating neurons. Interestingly, we also identified a role for both c-Jun and enhanced neuronal stress during initial neuronal infection in promoting a more reactivation-competent form of HSV-1 latency. Therefore, c-Jun functions at multiple stages during the HSV latent infection of neurons to promote reactivation but not during the initial JNK-dependent Phase I. Importantly, by demonstrating that initial infection conditions can contribute to later reactivation abilities, this study highlights the potential for latently infected neurons to maintain a molecular scar of previous exposure to neuronal stressors.IMPORTANCEThe molecular mechanisms that regulate the reactivation of herpes simplex virus-1 (HSV-1) from latent infection are unknown. The host transcription and pioneer factor c-Jun is the main target of the JNK cell stress pathway that is known to be important in exit of HSV from latency. Surprisingly, we found that c-Jun does not act with JNK during exit from latency but instead promotes the transition to full reactivation. Moreover, c-Jun and enhanced neuronal stress during initial neuronal infection promoted a more reactivation-competent form of HSV-1 latency. c-Jun, therefore, functions at multiple stages during HSV-1 latent infection of neurons to promote reactivation. Importantly, this study contributes to a growing body of evidence that de novo HSV-1 infection conditions can modulate latent infection and impact future reactivation events, raising important questions on the clinical impact of stress during initial HSV-1 acquisition on future reactivation events and consequences.
Keywords: c-Jun; herpes simplex virus; reactivation; stress response.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



Update of
-
c-Jun Signaling During Initial HSV-1 Infection Modulates Latency to Enhance Later Reactivation in addition to Directly Promoting the Progression to Full Reactivation.bioRxiv [Preprint]. 2023 Nov 10:2023.11.10.566462. doi: 10.1101/2023.11.10.566462. bioRxiv. 2023. Update in: J Virol. 2024 Feb 20;98(2):e0176423. doi: 10.1128/jvi.01764-23. PMID: 37986840 Free PMC article. Updated. Preprint.
Similar articles
-
Neuronal expression of herpes simplex virus-1 VP16 protein induces pseudorabies virus escape from silencing and reactivation.J Virol. 2024 Jul 23;98(7):e0056124. doi: 10.1128/jvi.00561-24. Epub 2024 Jun 13. J Virol. 2024. PMID: 38869285 Free PMC article.
-
c-Jun Signaling During Initial HSV-1 Infection Modulates Latency to Enhance Later Reactivation in addition to Directly Promoting the Progression to Full Reactivation.bioRxiv [Preprint]. 2023 Nov 10:2023.11.10.566462. doi: 10.1101/2023.11.10.566462. bioRxiv. 2023. Update in: J Virol. 2024 Feb 20;98(2):e0176423. doi: 10.1128/jvi.01764-23. PMID: 37986840 Free PMC article. Updated. Preprint.
-
DLK-Dependent Biphasic Reactivation of Herpes Simplex Virus Latency Established in the Absence of Antivirals.J Virol. 2022 Jun 22;96(12):e0050822. doi: 10.1128/jvi.00508-22. Epub 2022 May 24. J Virol. 2022. PMID: 35608347 Free PMC article.
-
Restarting Lytic Gene Transcription at the Onset of Herpes Simplex Virus Reactivation.J Virol. 2017 Jan 3;91(2):e01419-16. doi: 10.1128/JVI.01419-16. Print 2017 Jan 15. J Virol. 2017. PMID: 27807236 Free PMC article. Review.
-
A comparison of herpes simplex virus type 1 and varicella-zoster virus latency and reactivation.J Gen Virol. 2015 Jul;96(Pt 7):1581-602. doi: 10.1099/vir.0.000128. Epub 2015 Mar 20. J Gen Virol. 2015. PMID: 25794504 Free PMC article. Review.
Cited by
-
Herpesvirus initiation of dementias and autoimmune diseases.J Neurovirol. 2025 Jul 7. doi: 10.1007/s13365-025-01265-8. Online ahead of print. J Neurovirol. 2025. PMID: 40622506 Review.
-
Co-option of mitochondrial nucleic acid-sensing pathways by HSV-1 UL12.5 for reactivation from latent infection.Proc Natl Acad Sci U S A. 2025 Jan 28;122(4):e2413965122. doi: 10.1073/pnas.2413965122. Epub 2025 Jan 24. Proc Natl Acad Sci U S A. 2025. PMID: 39854226 Free PMC article.
-
Neuronal expression of herpes simplex virus-1 VP16 protein induces pseudorabies virus escape from silencing and reactivation.J Virol. 2024 Jul 23;98(7):e0056124. doi: 10.1128/jvi.00561-24. Epub 2024 Jun 13. J Virol. 2024. PMID: 38869285 Free PMC article.
-
A review of HSV pathogenesis, vaccine development, and advanced applications.Mol Biomed. 2024 Aug 29;5(1):35. doi: 10.1186/s43556-024-00199-7. Mol Biomed. 2024. PMID: 39207577 Free PMC article. Review.
-
Co-option of mitochondrial nucleic acid sensing pathways by HSV-1 UL12.5 for reactivation from latent Infection.bioRxiv [Preprint]. 2024 Jul 9:2024.07.06.601241. doi: 10.1101/2024.07.06.601241. bioRxiv. 2024. Update in: Proc Natl Acad Sci U S A. 2025 Jan 28;122(4):e2413965122. doi: 10.1073/pnas.2413965122. PMID: 39005440 Free PMC article. Updated. Preprint.
References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

