Mucosal correlates of protection after influenza viral challenge of vaccinated and unvaccinated healthy volunteers
- PMID: 38193710
- PMCID: PMC10865821
- DOI: 10.1128/mbio.02372-23
Mucosal correlates of protection after influenza viral challenge of vaccinated and unvaccinated healthy volunteers
Abstract
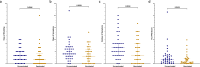
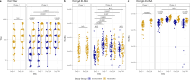
The induction of systemic antibody titers against hemagglutinin has long been the main focus of influenza vaccination strategies, but mucosal immunity has also been shown to play a key role in the protection against respiratory viruses. By vaccinating and challenging healthy volunteers, we demonstrated that inactivated influenza vaccine (IIV) modestly reduced the rate of influenza while predominantly boosting serum antibody titers against hemagglutinin (HA) and HA stalk, a consequence of the low neuraminidase (NA) content of IIV and the intramuscular route of administration. The viral challenge induced nasal and serum responses against both HA and NA. Correlations between mucosal IgA and serum IgG against specific antigens were low, whether before or after challenge, suggesting a compartmentalization of immune responses. Even so, volunteers who developed viral shedding for multiple days had lower baseline titers across both systemic and mucosal compartments as compared to those with no shedding or a single day of shedding. Regression analysis showed that pre-challenge HA inhibition titers were the most consistent correlate of protection across clinical outcomes combining shedding and symptoms, with NA inhibition titers and HA IgG levels only predicting the duration of shedding. Despite the inclusion of data from multiple binding and functional antibody assays against HA and NA performed on both serum and nasal samples, multivariate models were unable to account for the variability in outcomes, emphasizing our imperfect understanding of immune correlates in influenza and the importance of refining models with assessments of innate and cellular immune responses.IMPORTANCEThe devastating potential of influenza has been well known for over 100 years. Despite the development of vaccines since the middle of the 20th century, influenza continues to be responsible for substantial global morbidity and mortality. To develop next-generation vaccines with enhanced effectiveness, we must synthesize our understanding of the complex immune mechanisms culminating in protection. Our study outlines the differences in immune responses to influenza vaccine and influenza infection, identifying potential gaps in vaccine-induced immunity, particularly at the level of the nasal mucosa. Furthermore, this research underscores the need to refine our imperfect models while recognizing potential pitfalls in past and future attempts to identify and measure correlates of protection.
Keywords: correlates of protection; human challenge; influenza; influenza vaccines; mucosal immunity.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



Comment in
-
Unravelling influenza correlates of protection: lessons from human A/H1N1 Challenge.mBio. 2024 May 8;15(5):e0006424. doi: 10.1128/mbio.00064-24. Epub 2024 Mar 28. mBio. 2024. PMID: 38546212 Free PMC article.
References
-
- Memoli MJ, Shaw PA, Han A, Czajkowski L, Reed S, Athota R, Bristol T, Fargis S, Risos K, Powers JH, Davey RT, Taubenberger JK. 2016. Evaluation of antihemagglutinin and antineuraminidase antibodies as correlates of protection in an influenza A/H1N1 virus healthy human challenge model. mBio 7:e00417–16. doi:10.1128/mBio.00417-16 - DOI - PMC - PubMed
-
- Maier HE, Nachbagauer R, Kuan G, Ng S, Lopez R, Sanchez N, Stadlbauer D, Gresh L, Schiller A, Rajabhathor A, Ojeda S, Guglia AF, Amanat F, Balmaseda A, Krammer F, Gordon A. 2020. Pre-existing antineuraminidase antibodies are associated with shortened duration of influenza A(H1N1)pdm virus shedding and illness in naturally infected adults. Clin Infect Dis 70:2290–2297. doi:10.1093/cid/ciz639 - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous
