The oral administration of Lacticaseibacillus casei Shirota alleviates acetaminophen-induced liver injury through accelerated acetaminophen metabolism via the liver-gut axis in mice
- PMID: 38193757
- PMCID: PMC10826347
- DOI: 10.1128/msphere.00672-23
The oral administration of Lacticaseibacillus casei Shirota alleviates acetaminophen-induced liver injury through accelerated acetaminophen metabolism via the liver-gut axis in mice
Abstract
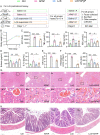
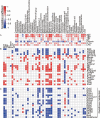
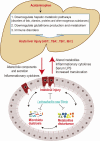
Acetaminophen is a widely used antipyretic and analgesic drug, and its overdose is the leading cause of drug-induced acute liver failure. This study aimed to investigate the effect and mechanism of Lacticaseibacillus casei Shirota (LcS), an extensively used and highly studied probiotic, on acetaminophen-induced acute liver injury. C57BL/6 mice were gavaged with LcS suspension or saline once daily for 7 days before acute liver injury was induced via intraperitoneal injection of 300 mg/kg acetaminophen. The results showed that LcS significantly decreased acetaminophen-induced liver and ileum injury, as demonstrated by reductions in the increases in aspartate aminotransferase, total bile acids, total bilirubin, indirect bilirubin, and hepatic cell necrosis. Moreover, LcS alleviated acetaminophen-induced intestinal mucosal permeability, decreased serum IL-1α and lipopolysaccharide levels, and elevated serum eosinophil chemokine (eotaxin) and hepatic glutathione levels. Furthermore, analysis of the gut microbiota and metabolome showed that LcS reduced the acetaminophen-enriched levels of Cyanobacteria, Oxyphotobacteria, long-chain fatty acids, cholesterol, and sugars in the gut. Additionally, the transcriptomic and proteomic results showed that LcS mitigated the decrease in metabolic and immune pathways as well as glutathione formation during acetaminophen-induced acute liver injury. This is the first study showing that pretreatment with LcS alleviates acetaminophen-enriched acute liver injury, and it provides a reference for the application of LcS.IMPORTANCEAcetaminophen is the most frequently used antipyretic analgesic worldwide. As a result, overdoses easily occur and lead to drug-induced acute liver injury, which quickly progresses to liver failure with a mortality of 60%-80% if not corrected in time. The current emergency treatment for overused acetaminophen needs to be administered within 8 hours to avoid liver injury or even liver failure. Therefore, developing preventive strategies for liver injury during planned acetaminophen medication is particularly important, preferably nonpharmacological methods. Lacticaseibacillus casei Shirota (LcS) is a famous probiotic that has been used for many years. Our study found that LcS significantly alleviated acetaminophen-induced acute liver injury, especially acetaminophen-induced liver injury toward fulminant hepatic failure. Here, we elucidated the function and potential mechanisms of LcS in alleviating acetaminophen-induced acute liver injury, hoping it will provide preventive strategies to people during acetaminophen treatment.
Keywords: Lacticaseibacillus casei Shirota; acetaminophen; glutathione; liver injury; liver-gut axis.
Conflict of interest statement
The authors declare no conflict of interest.
Figures





Similar articles
-
Probiotic Lactobacillus casei Shirota prevents acute liver injury by reshaping the gut microbiota to alleviate excessive inflammation and metabolic disorders.Microb Biotechnol. 2022 Jan;15(1):247-261. doi: 10.1111/1751-7915.13750. Epub 2021 Jan 25. Microb Biotechnol. 2022. PMID: 33492728 Free PMC article.
-
Metagenomic and proteomic approaches in elucidating aflatoxin B1 detoxification mechanisms of probiotic Lactobacillus casei Shirota towards intestine.Food Chem Toxicol. 2022 Feb;160:112808. doi: 10.1016/j.fct.2022.112808. Epub 2022 Jan 5. Food Chem Toxicol. 2022. PMID: 34998910
-
Akkermansia muciniphila Ameliorates Acetaminophen-Induced Liver Injury by Regulating Gut Microbial Composition and Metabolism.Microbiol Spectr. 2022 Feb 23;10(1):e0159621. doi: 10.1128/spectrum.01596-21. Epub 2022 Feb 2. Microbiol Spectr. 2022. PMID: 35107323 Free PMC article.
-
Immunomodulation by treatment with Lactobacillus casei strain Shirota.Int J Food Microbiol. 1998 May 26;41(2):133-40. doi: 10.1016/s0168-1605(98)00046-4. Int J Food Microbiol. 1998. PMID: 9704862 Review.
-
Acetaminophen hepatotoxicity: An update.Curr Gastroenterol Rep. 1999 Feb-Mar;1(1):42-9. doi: 10.1007/s11894-999-0086-3. Curr Gastroenterol Rep. 1999. PMID: 10980926 Review.
Cited by
-
Current progress on the microbial therapies for acute liver failure.Front Microbiol. 2024 Oct 16;15:1452663. doi: 10.3389/fmicb.2024.1452663. eCollection 2024. Front Microbiol. 2024. PMID: 39479215 Free PMC article. Review.
-
Modulation of Paracetamol-Induced Hepatotoxicity by Acute and Chronic Ethanol Consumption in Mice: A Study Pilot.Toxics. 2024 Nov 27;12(12):857. doi: 10.3390/toxics12120857. Toxics. 2024. PMID: 39771072 Free PMC article.
References
-
- Xie J. 2013. Adverse reactions and analysis of acetaminophen. China Pract Med (Chinese) 8:127–128. doi:10.3969/j.issn.1673-7555.2013.29.103 - DOI
-
- Li Y, Lv L, Ye J, Fang D, Shi D, Wu W, Wang Q, Wu J, Yang L, Bian X, Jiang X, Jiang H, Yan R, Peng C, Li L. 2019. Bifidobacterium adolescentis CGMCC 15058 alleviates liver injury, enhances the intestinal barrier and modifies the gut microbiota in D-galactosamine-treated rats. Appl Microbiol Biotechnol 103:375–393. doi:10.1007/s00253-018-9454-y - DOI - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

