Present and Future Innovations in AI and Cardiac MRI
- PMID: 38193835
- PMCID: PMC10831479
- DOI: 10.1148/radiol.231269
Present and Future Innovations in AI and Cardiac MRI
Abstract
Cardiac MRI is used to diagnose and treat patients with a multitude of cardiovascular diseases. Despite the growth of clinical cardiac MRI, complicated image prescriptions and long acquisition protocols limit the specialty and restrain its impact on the practice of medicine. Artificial intelligence (AI)-the ability to mimic human intelligence in learning and performing tasks-will impact nearly all aspects of MRI. Deep learning (DL) primarily uses an artificial neural network to learn a specific task from example data sets. Self-driving scanners are increasingly available, where AI automatically controls cardiac image prescriptions. These scanners offer faster image collection with higher spatial and temporal resolution, eliminating the need for cardiac triggering or breath holding. In the future, fully automated inline image analysis will most likely provide all contour drawings and initial measurements to the reader. Advanced analysis using radiomic or DL features may provide new insights and information not typically extracted in the current analysis workflow. AI may further help integrate these features with clinical, genetic, wearable-device, and "omics" data to improve patient outcomes. This article presents an overview of AI and its application in cardiac MRI, including in image acquisition, reconstruction, and processing, and opportunities for more personalized cardiovascular care through extraction of novel imaging markers.
© RSNA, 2024 See also the review "How AI May Transform Musculoskeletal Imaging" by Guermazi et al in this issue.
Conflict of interest statement
Figures




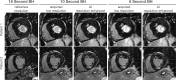
![Diagram shows artificial intelligence (AI)–enabled cardiac MRI
analysis, interpretation, and reporting. In cardiac MRI, images are
collected to characterize function (ie, cine and tagging images), flow,
tissue properties and fibrosis (ie, T1 and T2 maps and T1-weighted,
T2-weighted, and extracellular volume [ECV] images), and scarring (ie, late
gadolinium enhancement [LGE] images). AI can substantially impact the
analysis workflow after imaging. The clinical reading includes
quantification (eg, function on cine images) and clinical interpretation of
individual sequences (eg, presence and location of scarring on LGE images).
AI can assist in both the analysis and interpretation of clinical readings
and can change the role of imagers to oversight.](https://cdn.ncbi.nlm.nih.gov/pmc/blobs/3fb5/10831479/ffc0a91dc0a1/radiol.231269.fig9.gif)

References
-
- Guo R , Weingärtner S , Šiurytė P , et al. . Emerging techniques in cardiac magnetic resonance imaging . J Magn Reson Imaging 2022. ; 55 ( 4 ): 1043 – 1059 . - PubMed
-
- Rajiah PS , François CJ , Leiner T . Cardiac MRI: state of the art . Radiology 2023. ; 307 ( 3 ): e223008 . - PubMed
-
- Artificial intelligence and machine learning (AI/ML)-enabled medical devices . U.S. Food and Drug Administration . https://www.fda.gov/medical-devices/software-medical-device-samd/artific.... Updated October 19, 2023. Accessed October 19, 2023 .
-
- Ronneberger O , Fischer P , Brox T . U-Net: convolutional networks for biomedical image segmentation . In: Navab N , Hornegger J , Wells WM , Frangi AF , eds. Medical image computing and computer-assisted intervention—MICCAI 2015. Part III . Cham: : Springer International Publishing; , 2015. ; 234 – 241 .
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

