Therapeutic management of metabolic dysfunction associated steatotic liver disease
- PMID: 38193865
- PMCID: PMC10954426
- DOI: 10.1002/ueg2.12525
Therapeutic management of metabolic dysfunction associated steatotic liver disease
Abstract
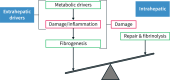
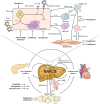
The incidence and prevalence of non-alcoholic fatty liver disease (NAFLD) have been steadily increasing worldwide, with a huge societal and economic burden. Recently, NAFLD and non-alcoholic steatohepatitis have been renamed and redefined as metabolic dysfunction associated steatotic liver disease (MASLD) and steatohepatitis (Metabolic Dysfunction Associated Steatohepatitis (MASH)), which result from an imbalance between metabolic and inflammatory stress (mainly as a consequence of adipose tissue dysfunction and insulin resistance) and the defence and repair mechanisms of the steatotic liver. Once MASLD progresses to end-stage of liver disease, treatment efficacy becomes limited and may require liver transplantation. Early detection and intervention are crucial. Lifestyle modification is consequently the cornerstone of its management. Timely consideration of bariatric surgeries should be given to patients meeting specific criteria. A multidisciplinary approach is warranted, starting from the concept that MASLD/MASH is at the centre of the cardiovascular-liver-metabolic syndrome. In some cases, pharmacological treatment can complement lifestyle modification. Several drugs used to treat the cardiometabolic co-morbidities have some potential efficacy in slowing Down disease progression, and some have demonstrated efficacy on histological endpoints that are likely to translate into long-term clinical benefits. Optimising the use of these drugs within their licenced indications is thus paramount for patients with MASLD. Several MASH-specific drugs are on the horizon and are likely to enrich our therapeutic armamentarium in the near future, particularly in non-cirrhotic stages of the disease. Much work still needs to be done to understand the specific features of MASH cirrhosis and develop efficacious treatments for this disease stage.
Keywords: MASH; MASLD; NAFLD; NASH; management; metabolic dysfunction associated steatotic liver disease; metabolic syndrome lifestyle; therapy.
© 2024 The Authors. United European Gastroenterology Journal published by Wiley Periodicals LLC on behalf of United European Gastroenterology.
Conflict of interest statement
SF holds a senior clinical investigator fellowship from the Research Foundation Flanders (FWO) (1802154N). His institution has received grants from Astellas, Falk Pharma, Genfit, Gilead Sciences, GlympsBio, Janssens Pharmaceutica, Inventiva, Merck Sharp & Dome, Pfizer, and Roche. He has acted as consultant for Abbvie, Actelion, Aelin Therapeutics, AgomAb, Aligos Therapeutics, Allergan, Alnylam, Astellas, Astra Zeneca, Bayer, Boehringer Ingelheim, Bristoll‐Meyers Squibb, CSL Behring, Coherus, Echosens, Dr. Falk Pharma, Eisai, Enyo, Galapagos, Galmed, Genetech, Genfit, Genflow Biosciences, Gilead Sciences, Intercept, Inventiva, Janssens Pharmaceutica, PRO.MED.CS Praha, Julius Clinical, Madrigal, Medimmune, Merck Sharp & Dome, Mursla Bio, NGM Bio, Novartis, Novo Nordisk, Promethera, Roche, Siemens Healthineers. He has been a lecturer for Abbvie, Allergan, Bayer, Eisai, Genfit, Gilead Sciences, Janssens Cilag, Intercept, Inventiva, Merck Sharp & Dome, Novo Nordisk, Promethera, and Siemens Healthineers.
Figures

Comment in
-
Reasons why treatment of metabolic dysfunction-associated steatotic liver disease may benefit from targeting the gut-liver axis as well.United European Gastroenterol J. 2024 May;12(4):528-529. doi: 10.1002/ueg2.12558. Epub 2024 Mar 27. United European Gastroenterol J. 2024. PMID: 38544364 Free PMC article. No abstract available.
References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

