Increased LL37 in psoriasis and other inflammatory disorders promotes LDL uptake and atherosclerosis
- PMID: 38194294
- PMCID: PMC10904043
- DOI: 10.1172/JCI172578
Increased LL37 in psoriasis and other inflammatory disorders promotes LDL uptake and atherosclerosis
Abstract
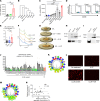
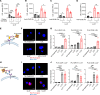
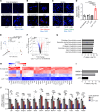
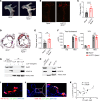
Patients with chronic inflammatory disorders such as psoriasis have an increased risk of cardiovascular disease and elevated levels of LL37, a cathelicidin host defense peptide that has both antimicrobial and proinflammatory properties. To explore whether LL37 could contribute to the risk of heart disease, we examined its effects on lipoprotein metabolism and show that LL37 enhanced LDL uptake in macrophages through the LDL receptor (LDLR), scavenger receptor class B member 1 (SR-B1), and CD36. This interaction led to increased cytosolic cholesterol in macrophages and changes in expression of lipid metabolism genes consistent with increased cholesterol uptake. Structure-function analysis and synchrotron small-angle x-ray scattering showed structural determinants of the LL37-LDL complex that underlie its ability to bind its receptors and promote uptake. This function of LDL uptake is unique to cathelicidins from humans and some primates and was not observed with cathelicidins from mice or rabbits. Notably, Apoe-/- mice expressing LL37 developed larger atheroma plaques than did control mice, and a positive correlation between plasma LL37 and oxidized phospholipid on apolipoprotein B (OxPL-apoB) levels was observed in individuals with cardiovascular disease. These findings provide evidence that LDL uptake can be increased via interaction with LL37 and may explain the increased risk of cardiovascular disease associated with chronic inflammatory disorders.
Keywords: Atherosclerosis; Cardiology; Dermatology; Innate immunity; Skin.
Conflict of interest statement
Figures

References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

