A structurally precise mechanism links an epilepsy-associated KCNC2 potassium channel mutation to interneuron dysfunction
- PMID: 38194456
- PMCID: PMC10801864
- DOI: 10.1073/pnas.2307776121
A structurally precise mechanism links an epilepsy-associated KCNC2 potassium channel mutation to interneuron dysfunction
Abstract
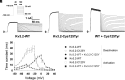
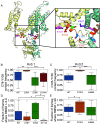
De novo heterozygous variants in KCNC2 encoding the voltage-gated potassium (K+) channel subunit Kv3.2 are a recently described cause of developmental and epileptic encephalopathy (DEE). A de novo variant in KCNC2 c.374G > A (p.Cys125Tyr) was identified via exome sequencing in a patient with DEE. Relative to wild-type Kv3.2, Kv3.2-p.Cys125Tyr induces K+ currents exhibiting a large hyperpolarizing shift in the voltage dependence of activation, accelerated activation, and delayed deactivation consistent with a relative stabilization of the open conformation, along with increased current density. Leveraging the cryogenic electron microscopy (cryo-EM) structure of Kv3.1, molecular dynamic simulations suggest that a strong π-π stacking interaction between the variant Tyr125 and Tyr156 in the α-6 helix of the T1 domain promotes a relative stabilization of the open conformation of the channel, which underlies the observed gain of function. A multicompartment computational model of a Kv3-expressing parvalbumin-positive cerebral cortex fast-spiking γ-aminobutyric acidergic (GABAergic) interneuron (PV-IN) demonstrates how the Kv3.2-Cys125Tyr variant impairs neuronal excitability and dysregulates inhibition in cerebral cortex circuits to explain the resulting epilepsy.
Keywords: KCNC2; Kv3.2; epilepsy; neurogenetics; potassium channels.
Conflict of interest statement
Competing interests statement:The authors declare no competing interest.
Figures



References
-
- Gutman G. A., et al. , International union of pharmacology. XLI. Compendium of voltage-gated ion channels: Potassium channels. Pharmacol. Rev. 55, 583–586 (2003). - PubMed
-
- Lindy A. S., et al. , Diagnostic outcomes for genetic testing of 70 genes in 8565 patients with epilepsy and neurodevelopmental disorders. Epilepsia 59, 1062–1071 (2018). - PubMed
-
- Rudy B., McBain C. J., Kv3 channels: Voltage-gated K+ channels designed for high-frequency repetitive firing. Trends Neurosci. 24, 517–526 (2001). - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

