Rombocin, a Short Stable Natural Nisin Variant, Displays Selective Antimicrobial Activity against Listeria monocytogenes and Employs a Dual Mode of Action to Kill Target Bacterial Strains
- PMID: 38194633
- PMCID: PMC10804407
- DOI: 10.1021/acssynbio.3c00612
Rombocin, a Short Stable Natural Nisin Variant, Displays Selective Antimicrobial Activity against Listeria monocytogenes and Employs a Dual Mode of Action to Kill Target Bacterial Strains
Abstract
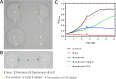
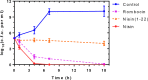
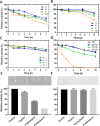
Nisin, with its unique mode of action and potent antimicrobial activity, serves as a remarkable inspiration for the design of novel antibiotics. However, peptides possess inherent weaknesses, particularly their susceptibility to proteolytic degradation, such as by trypsin, which limits their broader applications. This led us to speculate that natural variants of nisin produced by underexplored bacterial species can potentially overcome these limitations. We carried out genome mining of two Romboutsia sedimentorum strains, RC001 and RC002, leading to the discovery of rombocin A, which is a 25 amino acid residue short nisin variant that is predicted to have only four macrocycles compared to the known 31-35 amino acids long nisin variants with five macrocycles. Using the nisin-controlled expression system, we heterologously expressed fully modified and functional rombocin A in Lactococcus lactis and demonstrated its selective antimicrobial activity against Listeria monocytogenes. Rombocin A uses a dual mode of action involving lipid II binding activity and dissipation of the membrane potential to kill target bacteria. Stability tests confirmed its high stability at different pH values, temperatures, and in particular, against enzymatic degradation. With its gene-encoded characteristic, rombocin A is amenable to bioengineering to generate novel derivatives. Further mutation studies led to the identification of rombocin K, a mutant with enhanced bioactivity against L. monocytogenes. Our findings suggest that rombocin A and its bioengineered variant, rombocin K, are promising candidates for development as food preservatives or antibiotics against L. monocytogenes.
Keywords: four lanthionine rings; mode of action; mutagenesis; short nisin variant; specificity; stability.
Conflict of interest statement
The authors declare no competing financial interest.
Figures





Similar articles
-
Altering Specificity and Enhancing Stability of the Antimicrobial Peptides Nisin and Rombocin through Dehydrated Amino Acid Residue Engineering.Peptides. 2024 Apr;174:171152. doi: 10.1016/j.peptides.2024.171152. Epub 2024 Jan 12. Peptides. 2024. PMID: 38220092
-
Engineering hybrid lantibiotics yields the highly stable and bacteriocidal peptide cerocin V.Microbiol Res. 2024 May;282:127640. doi: 10.1016/j.micres.2024.127640. Epub 2024 Feb 9. Microbiol Res. 2024. PMID: 38350171
-
Investigation of combinations of rationally selected bioengineered nisin derivatives for their ability to inhibit Listeria in broth and model food systems.Food Microbiol. 2021 Oct;99:103835. doi: 10.1016/j.fm.2021.103835. Epub 2021 May 20. Food Microbiol. 2021. PMID: 34119119
-
Bioengineering of the model lantibiotic nisin.Bioengineered. 2015;6(4):187-92. doi: 10.1080/21655979.2015.1049781. Epub 2015 May 13. Bioengineered. 2015. PMID: 25970137 Free PMC article. Review.
-
Nisin variants: What makes them different and unique?Peptides. 2024 Jul;177:171220. doi: 10.1016/j.peptides.2024.171220. Epub 2024 Apr 16. Peptides. 2024. PMID: 38636811 Review.
Cited by
-
Bacteriocin diversity, function, discovery and application as antimicrobials.Nat Rev Microbiol. 2024 Sep;22(9):556-571. doi: 10.1038/s41579-024-01045-x. Epub 2024 May 10. Nat Rev Microbiol. 2024. PMID: 38730101 Free PMC article. Review.
-
An Engineered Nisin Analogue with a Hydrophobic Moiety Attached at Position 17 Selectively Inhibits Enterococcus faecium Strains.ACS Chem Biol. 2024 Sep 20;19(9):2023-2031. doi: 10.1021/acschembio.4c00337. Epub 2024 Sep 10. ACS Chem Biol. 2024. PMID: 39254256 Free PMC article.
-
Promiscuity of lanthipeptide enzymes: new challenges and applications.World J Microbiol Biotechnol. 2025 Aug 6;41(8):298. doi: 10.1007/s11274-025-04505-5. World J Microbiol Biotechnol. 2025. PMID: 40768108 Free PMC article. Review.
-
Facile Halogenation of Antimicrobial Peptides As Demonstrated by Producing Bromotryptophan-Labeled Nisin Variants with Enhanced Antimicrobial Activity.J Nat Prod. 2024 Jun 28;87(6):1548-1555. doi: 10.1021/acs.jnatprod.4c00118. Epub 2024 Jun 18. J Nat Prod. 2024. PMID: 38888620 Free PMC article.
References
-
- Montalbán-López M.; Scott T. A.; Ramesh S.; Rahman I. R.; van Heel A. J.; Viel J. H.; Bandarian V.; Dittmann E.; Genilloud O.; Goto Y.; Grande Burgos M. J.; Hill C.; Kim S.; Koehnke J.; Latham J. A.; Link A. J.; Martínez B.; Nair S. K.; Nicolet Y.; Rebuffat S.; Sahl H.-G.; Sareen D.; Schmidt E. W.; Schmitt L.; Severinov K.; Süssmuth R. D.; Truman A. W.; Wang H.; Weng J.-K.; van Wezel G. P.; Zhang Q.; Zhong J.; Piel J.; Mitchell D. A.; Kuipers O. P.; van der Donk W. A. New developments in RiPP discovery, enzymology and engineering. Nat. Prod. Rep. 2021, 38 (1), 130–239. 10.1039/D0NP00027B. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

