Clinical and functional spectrum of RAC2-related immunodeficiency
- PMID: 38194689
- PMCID: PMC11033590
- DOI: 10.1182/blood.2023022098
Clinical and functional spectrum of RAC2-related immunodeficiency
Erratum in
-
Donkó A, Sharapova SO, Kabat J, et al. Clinical and functional spectrum of RAC2-related immunodeficiency. Blood. 2024;143(15):1476-1487.Blood. 2024 Sep 5;144(10):1132. doi: 10.1182/blood.2024025827. Blood. 2024. PMID: 39235795 Free PMC article. No abstract available.
Abstract
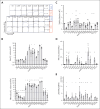
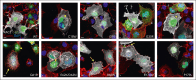
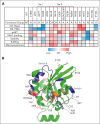
Mutations in the small Rho-family guanosine triphosphate hydrolase RAC2, critical for actin cytoskeleton remodeling and intracellular signal transduction, are associated with neonatal severe combined immunodeficiency (SCID), infantile neutrophilic disorder resembling leukocyte adhesion deficiency (LAD), and later-onset combined immune deficiency (CID). We investigated 54 patients (23 previously reported) from 37 families yielding 15 novel RAC2 missense mutations, including one present only in homozygosity. Data were collected from referring physicians and literature reports with updated clinical information. Patients were grouped by presentation: neonatal SCID (n = 5), infantile LAD-like disease (n = 5), or CID (n = 44). Disease correlated to RAC2 activity: constitutively active RAS-like mutations caused neonatal SCID, dominant-negative mutations caused LAD-like disease, whereas dominant-activating mutations caused CID. Significant T- and B-lymphopenia with low immunoglobulins were seen in most patients; myeloid abnormalities included neutropenia, altered oxidative burst, impaired neutrophil migration, and visible neutrophil macropinosomes. Among 42 patients with CID with clinical data, upper and lower respiratory infections and viral infections were common. Twenty-three distinct RAC2 mutations, including 15 novel variants, were identified. Using heterologous expression systems, we assessed downstream effector functions including superoxide production, p21-activated kinase 1 binding, AKT activation, and protein stability. Confocal microscopy showed altered actin assembly evidenced by membrane ruffling and macropinosomes. Altered protein localization and aggregation were observed. All tested RAC2 mutant proteins exhibited aberrant function; no single assay was sufficient to determine functional consequence. Most mutants produced elevated superoxide; mutations unable to support superoxide formation were associated with bacterial infections. RAC2 mutations cause a spectrum of immune dysfunction, ranging from early onset SCID to later-onset combined immunodeficiencies depending on RAC2 activity. This trial was registered at www.clinicaltrials.gov as #NCT00001355 and #NCT00001467.
Conflict of interest statement
Conflict-of-interest disclosure: S.K. is a Centre National de la Recherche Scientifique staff researcher. J.W. is a consultant on the Advisory Board of Octapharma, X4-Pharmaceuticals, Pharming, CSL-Behring, Takeda, and Enzyvant. J.W. is a medical writer for UptoDate. The remaining authors declare no competing financial interests.
Figures

Comment in
-
Rac'n'rolling the immune system.Blood. 2024 Apr 11;143(15):1433-1434. doi: 10.1182/blood.2023023630. Blood. 2024. PMID: 38602700 No abstract available.
References
-
- Knaus UG, Heyworth PG, Evans T, Curnutte JT, Bokoch GM. Regulation of phagocyte oxygen radical production by the GTP-binding protein Rac2. Science. 1991;254(5037):1512–1515. - PubMed
-
- Edwards DC, Sanders LC, Bokoch GM, Gill GN. Activation of LIM-kinase by Pak1 couples Rac/Cdc42 GTPase signalling to actin cytoskeletal dynamics. Nat Cell Biol. 1999;1(5):253–259. - PubMed
-
- Williams DA, Tao W, Yang F, et al. Dominant negative mutation of the hematopoietic-specific Rho GTPase, Rac2, is associated with a human phagocyte immunodeficiency. Blood. 2000;96(5):1646–1654. - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

