Durable response after tisagenlecleucel in adults with relapsed/refractory follicular lymphoma: ELARA trial update
- PMID: 38194692
- PMCID: PMC11103095
- DOI: 10.1182/blood.2023021567
Durable response after tisagenlecleucel in adults with relapsed/refractory follicular lymphoma: ELARA trial update
Abstract
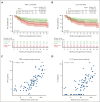
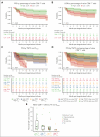
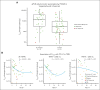
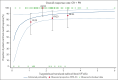
Tisagenlecleucel is approved for adults with relapsed/refractory (r/r) follicular lymphoma (FL) in the third- or later-line setting. The primary analysis (median follow-up, 17 months) of the phase 2 ELARA trial reported high response rates and excellent safety profile in patients with extensively pretreated r/r FL. Here, we report longer-term efficacy, safety, pharmacokinetic, and exploratory biomarker analyses after median follow-up of 29 months (interquartile range, 22.2-37.7). As of 29 March 2022, 97 patients with r/r FL (grades 1-3A) received tisagenlecleucel infusion (0.6 × 108-6 × 108 chimeric antigen receptor-positive viable T cells). Bridging chemotherapy was allowed. Baseline clinical factors, tumor microenvironment, blood soluble factors, and circulating blood cells were correlated with clinical response. Cellular kinetics were assessed by quantitative polymerase chain reaction. Median progression-free survival (PFS), duration of response (DOR), and overall survival (OS) were not reached. Estimated 24-month PFS, DOR, and OS rates in all patients were 57.4% (95% confidence interval [CI], 46.2-67), 66.4% (95% CI, 54.3-76), and 87.7% (95% CI, 78.3-93.2), respectively. Complete response rate and overall response rate were 68.1% (95% CI, 57.7-77.3) and 86.2% (95% CI, 77.5-92.4), respectively. No new safety signals or treatment-related deaths were reported. Low levels of tumor-infiltrating LAG3+CD3+ exhausted T cells and higher baseline levels of naïve CD8+ T cells were associated with improved outcomes. Tisagenlecleucel continued to demonstrate highly durable efficacy and a favorable safety profile in this extended follow-up of 29 months in patients with r/r FL enrolled in ELARA. This trial was registered at www.clinicaltrials.gov as #NCT03568461.
© 2024 American Society of Hematology. Published by Elsevier Inc. Licensed under Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International (CC BY-NC-ND 4.0), permitting only noncommercial, nonderivative use with attribution. All other rights reserved.
Conflict of interest statement
Conflicts-of-interest disclosure: M. Dreyling reports consulting for, or advisory role with, AbbVie, AstraZeneca, Bayer, BeiGene, Bristol Myers Squibb/Celgene, Genmab, Gilead Kite, Janssen, Lilly, Novartis, and Roche; is a member of the speakers’ bureau of AstraZeneca, Gilead Kite, Janssen, Lilly, Novartis, and Roche; and reports research funding from AbbVie, Bayer, Bristol Myers Squibb/Celgene, Gilead Kite, Janssen, and Roche. N.H.F. reports grants from Novartis and TG Therapeutics; and reports consulting for, or advisory role with, Bristol Myers Squibb, Gilead, and Roche. M. Dickinson received honoraria from Amgen, Bristol Myers Squibb, Gilead Sciences, Janssen, Novartis, Merck Sharp & Dohme, and Roche; reports consulting for, or advisory role with, Bristol Myers Squibb, Gilead Sciences, Janssen, Merck Sharp & Dohme, Novartis, and Roche; is a member of the speakers’ bureau of Gilead Sciences, Novartis, Merck Sharp & Dohme, and Roche; received research funding from Celgene, Novartis, Merck Sharp & Dohme, Roche, and Takeda; and received funds for travel, accommodations, and expenses from Roche. J.M.-L. reports consulting for, or advisory role with, Astellas, Bristol Myers Squibb, Incyte, Janssen, Novartis, and Roche; is a member of the speakers’ bureau of Astellas, Bristol Myers Squibb, Incyte, Janssen, Novartis, and Roche; and received research funding from Astellas, Bristol Myers Squibb, Incyte, and Roche. A.K. reports consulting for, or advisory role with, Nordic Nanovector; and received research funding from Nordic Nanovector. J.B. received honoraria from Janssen and Novartis; reports consulting for, or advisory role with, Gilead Sciences, Janssen, and Novartis; and is a member of the speakers’ bureau of Gilead Sciences, Novartis, Janssen, Roche, and Takeda. M.G. received research funding from Bristol Myers Squibb, Kite, Takeda, Fate, Atara, Incyte, and Miltenyi. L.P. received honoraria from Pfizer and Roche; and received funds for travel, accommodations, and expenses from Novartis. J.C.C. reports consulting for, or advisory role with, Bayer, Bristol Myers Squibb, Karyopharm Therapeutics, Kite/Gilead, MorphoSys, Novartis, Pfizer, Teneobio, Adicet Bio, ADC Therapeutics, and BeiGene; is a member of the speakers’ bureau of BeiGene; and received research funding from Merck, AstraZeneca, and Adaptive. E.B. reports grants from Amgen and Takeda; reports personal fees from Celgene, Gilead, Janssen, Novartis, and Roche; reports nonfinancial support from AbbVie, BeiGene, Celgene, and Roche; and reports consulting for, or advisory role with, Incyte and Roche. K.K. received honoraria from Bristol Myers Squibb, Celgene, Dainippon-Sumitomo, Janssen, Kyowa Kirin, Merck Sharp & Dohme, Mundi, and Ono; reports consulting for, or advisory role with, AbbVie, AstraZeneca, Celgene, Chugai Pharmaceutical, Daiichi Sankyo, Eisai, Janssen, and Novartis; and received research funding from AbbVie, Bristol Myers Squibb, Celgene, Chugai, Daiichi Sankyo, Eisai, Janssen, Kyowa Kirin, Novartis, and Ono. H.H. received honoraria from Bristol Myers Squibb, Chugai, Novartis, and Sanofi; and received research funding from Asahi Kasei Pharma, Chugai, Eisai, Kyowa Kirin, and Takeda. M.J.K. received honoraria from Bristol Myers Squibb/Celgene, Kite/Gilead, Novartis, and Roche; reports consulting for, or advisory role with, Bristol Myers Squibb/Celgene, Kite/Gilead, Miltenyi Biotech, Novartis, Takeda, Adicet Bio, and Miltenyi Biomedicine; and received research funding from Kite/Gilead. C.A. reports consulting for, or advisory role with, Atara Biotherapeutics, Epizyme, Gilead Sciences, Incyte, Karyopharm Therapeutics, Kite Pharma, and TG Therapeutics; received research funding from Amgen, Celgene, GlaxoSmithKline, Juno Therapeutics, Merck, and Novartis; and received funds for travel, accommodations, and expenses from Gilead Sciences and Kite Pharma. P.A.R. received honoraria from Novartis; reports consulting for, or advisory role with, AbbVie, Genmab, ADC Therapeutics, Pharmacyclics, Novartis, Bristol Myers Squibb, Kite/Gilead, Nurix Therapeutics, Nektar Therapeutics, Takeda, Intellia Therapeutics, Sana Biotechnology, BeiGene, Janssen, and CVS Caremark; is a member of the speakers’ bureau of Kite/Gilead; and received research funding from Bristol Myers Squibb, Kite/Gilead, Novartis, MorphoSys, CRISPR Therapeutics, Calibr, Xencor, Fate Therapeutics, and Tessa Therapeutics. J.A.P.-S. received honoraria from Alexion, Kite/Gilead, Novartis, Janssen, and Jazz; and is a member of the speakers’ bureau of, and/or received research funding from, Alexion, Kite/Gilead, Novartis, Bristol Myers Squibb, Amgen, Jazz, Takeda, Pfizer, and AbbVie. A.I.C. received honoraria from MorphoSys and Mesoblast. L.J.N. received honoraria from ADC Therapeutics, Bayer, Bristol Myers Squibb/Celgene, Denovo Pharma, Epizyme, Genentech, Gilead/Kite, Janssen, MorphoSys, Novartis, Pfizer, Takeda, and TG Therapeutics; and received research funding from Bristol Myers Squibb/Celgene, Caribou Biosciences, Epizyme, Genentech, Gilead/Kite, IGM Biosciences, Janssen, Novartis, Pfizer, Takeda, and TG Therapeutics. B.v.T. received honoraria from AstraZeneca, Bristol Myers Squibb, Incyte, Novartis, Roche Pharma AG, Takeda, and Merck Sharp & Dohme; reports consulting for, or advisory role with, Allogene, Bristol Myers Squibb/Celgene, Cerus, Incyte, IQVIA, Gilead Kite, Miltenyi, Novartis, Noscendo, PentixaPharm, Roche, Amgen, Pfizer, Takeda, Merck Sharp & Dohme, and Gilead Kite; received research funding from Merck Sharp & Dohme, Novartis, and Takeda; and received funds for travel, accommodations, and expenses from AbbVie, AstraZeneca, Gilead Kite, Merck Sharp & Dohme, Roche, Takeda, and Novartis. A.J.M.F. reports consulting for, or advisory role with, Gilead, Incyte, Novartis, PentixaPharm, and Roche; is a member of the speakers’ bureau of Adienne; received research funding from ADC Therapeutics, Amgen, BeiGene, Bristol Myers Squibb, Genmab, Gilead, Hutchison Medipharma, Novartis, Pharmacyclics, PentixaPharm, Pfizer, and Roche; and reports patents with Ospedale San Raffaele SRL. T.T. received honoraria from Bristol Myers Squibb, Kyowa Kirin, Merck Sharp & Dohme, Pfizer, and Takeda; reports consulting for, or advisory role with, Merck Sharp & Dohme, Novartis, and Takeda; received research funding from Astellas, Chugai Pharmaceutical, Fuji Pharma, Kyowa Kirin, Nippon Shinyaku, Novartis, Sanofi, and Teijin Pharma; and reports other manuscript support from Janssen and Novartis. P.E.M.P. received honoraria from AbbVie, AstraZeneca, BeiGene, Gilead Sciences, Janssen, and Novartis; and received research funding from Gilead Sciences and Roche; and reports consulting for, or advisory role with, AbbVie, BeiGene, and Novartis. J.P.M. is employed by the University of Kansas Medical Center; received honoraria from Juno Therapeutics, Kite Pharmaceuticals, and Magenta Therapeutics; reports consulting for, or advisory role with, AlloVir, EcoR1 CAPITAL, Juno Therapeutics, Kite Pharmaceuticals, Magenta Therapeutics, and Novartis; and received research funding from Juno Therapeutics and Kite Pharmaceuticals. A.L.P. received honoraria from Amgen, Celgene, Janssen, Kite/Gilead, and Novartis; reports consulting for, or advisory role with, Amgen, Celgene, Kite/Gilead, and Novartis; and is a member of the speakers’ bureau of Janssen. A.V. received honoraria from Amgen, Bristol Myers Squibb, Kite/Gilead, Novartis, and Roche; and reports consulting for, or advisory role with, Amgen, Bristol Myers Squibb, Kite/Gilead, Novartis, Roche, and AbbVie. P.L.Z. reports consulting for, or advisory role with, ADC Therapeutics, BeiGene, Bristol Myers Squibb, Celltrion, EUSA Pharma, Gilead, Incyte, Janssen-Cilag, Kyowa Kirin, Merck Sharp & Dohme, Novartis, Roche, Sandoz, Servier, TG Therapeutics, Takeda, and Verastem; and is a member of the speakers’ bureau of BeiGene, Bristol Myers Squibb, Celltrion, EUSA Pharma, Gilead, Incyte, Janssen-Cilag, Kyowa Kirin, Merck Sharp & Dohme, Novartis, Roche, Servier, TG Therapeutics, Takeda, and Verastem. R.M. received honoraria from Novartis; and reports consulting for, or advisory role with, Novartis. I.P., A.Z., R.A., X.H., D.G., and D.O. are employed by Novartis. R.R., H.J.M., and A.M. are employed by Novartis; and reports stock and other ownership interests in Novartis. C.T. received honoraria from ADC Therapeutics, BeiGene, Gilead, Novartis, Roche, and Takeda; reports consulting for, or advisory role with, BeiGene, Gilead, Incyte, Novartis, and Roche; and received funds for travel, accommodations, and expenses from Novartis. S.J.S. received honoraria from Incyte, Novartis, and Takeda; reports consulting for, or advisory role with, AstraZeneca, BeiGene, Celgene/Bristol Myers Squibb/Juno Therapeutics, Fate Therapeutics, Genentech/Roche, Genmab, Incyte, Janssen, Legend Biotech, MorphoSys, Mustang Biotech, Nordic Nanovector, Novartis, Regeneron, and Vittoria; reports grants from AbbVie, Acerta, AstraZeneca, Celgene/Bristol Myers Squibb/Juno Therapeutics, Genentech/Roche, Merck, Novartis, and TG Therapeutics; and reports patents with Novartis. P.J.H. and F.O. report no competing financial interests.
Figures



Comment in
-
A promising step for high-risk FL.Blood. 2024 Apr 25;143(17):1679-1681. doi: 10.1182/blood.2023023686. Blood. 2024. PMID: 38662385 No abstract available.
References
-
- Dreyling M, Ghielmini M, Rule S, et al. Newly diagnosed and relapsed follicular lymphoma: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2021;32(3):298–308. - PubMed
-
- Casulo C, Barr PM. How I treat early-relapsing follicular lymphoma. Blood. 2019;133(14):1540–1547. - PubMed
-
- Rivas-Delgado A, Magnano L, Moreno-Velázquez M, et al. Response duration and survival shorten after each relapse in patients with follicular lymphoma treated in the rituximab era. Br J Haematol. 2019;184(5):753–759. - PubMed
-
- Link BK, Day BM, Zhou X, et al. Second-line and subsequent therapy and outcomes for follicular lymphoma in the United States: data from the observational National LymphoCare Study. Br J Haematol. 2019;184(4):660–663. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
Research Materials

