Towards stratified treatment of JIA: machine learning identifies subtypes in response to methotrexate from four UK cohorts
- PMID: 38194741
- PMCID: PMC10792564
- DOI: 10.1016/j.ebiom.2023.104946
Towards stratified treatment of JIA: machine learning identifies subtypes in response to methotrexate from four UK cohorts
Abstract
Background: Methotrexate (MTX) is the gold-standard first-line disease-modifying anti-rheumatic drug for juvenile idiopathic arthritis (JIA), despite only being either effective or tolerated in half of children and young people (CYP). To facilitate stratified treatment of early JIA, novel methods in machine learning were used to i) identify clusters with distinct disease patterns following MTX initiation; ii) predict cluster membership; and iii) compare clusters to existing treatment response measures.
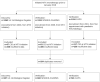
Methods: Discovery and verification cohorts included CYP who first initiated MTX before January 2018 in one of four UK multicentre prospective cohorts of JIA within the CLUSTER consortium. JADAS components (active joint count, physician (PGA) and parental (PGE) global assessments, ESR) were recorded at MTX start and over the following year. Clusters of MTX 'response' were uncovered using multivariate group-based trajectory modelling separately in discovery and verification cohorts. Clusters were compared descriptively to ACR Pedi 30/90 scores, and multivariate logistic regression models predicted cluster-group assignment.
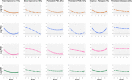
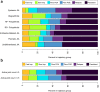
Findings: The discovery cohorts included 657 CYP and verification cohorts 1241 CYP. Six clusters were identified: Fast improvers (11%), Slow Improvers (16%), Improve-Relapse (7%), Persistent Disease (44%), Persistent PGA (8%) and Persistent PGE (13%), the latter two characterised by improvement in all features except one. Factors associated with clusters included ethnicity, ILAR category, age, PGE, and ESR scores at MTX start, with predictive model area under the curve values of 0.65-0.71. Singular ACR Pedi 30/90 scores at 6 and 12 months could not capture speeds of improvement, relapsing courses or diverging disease patterns.
Interpretation: Six distinct patterns following initiation of MTX have been identified using methods in artificial intelligence. These clusters demonstrate the limitations in traditional yes/no treatment response assessment (e.g., ACRPedi30) and can form the basis of a stratified medicine programme in early JIA.
Funding: Medical Research Council, Versus Arthritis, Great Ormond Street Hospital Children's Charity, Olivia's Vision, and the National Institute for Health Research.
Keywords: Epidemiology; Juvenile idiopathic arthritis; Machine learning; Methotrexate; Treatment outcome.
Copyright © 2024 The Authors. Published by Elsevier B.V. All rights reserved.
Conflict of interest statement
Declaration of interests The CLUSTER consortium reports grants from AbbVie and Sobi outside the submitted work, in addition to funding from Versus Arthritis (20747), the British Society for Rheumatology, Pfizer, Sparks UK (08ICH09), the Medical Research Council (MR/M004600/1), and UK Juvenile Idiopathic Arthritis Genetics Consortium for CLUSTER cohorts. KLH reports grants from BMS and Pfizer, and speaker's fees from AbbVie, outside the submitted work. All other authors declare no other competing interests.
Figures

References
-
- Ruperto N., Murray K.J., Gerloni V., et al. A randomized trial of parenteral methotrexate comparing an intermediate dose with a higher dose in children with juvenile idiopathic arthritis who failed to respond to standard doses of methotrexate. Arthritis Rheum. 2004;50(7):2191–2201. - PubMed
-
- Foell D., Wulffraat N., Wedderburn L.R., et al. Methotrexate withdrawal at 6 vs 12 months in juvenile idiopathic arthritis in remission: a randomized clinical trial. JAMA. 2010;303(13):1266–1273. - PubMed
-
- Vilca I., Munitis P.G., Pistorio A., et al. Predictors of poor response to methotrexate in polyarticular-course juvenile idiopathic arthritis: analysis of the PRINTO methotrexate trial. Ann Rheum Dis. 2010;69(8):1479–1483. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

