Temporal regulation of vegetative phase change in plants
- PMID: 38194910
- PMCID: PMC10783531
- DOI: 10.1016/j.devcel.2023.11.010
Temporal regulation of vegetative phase change in plants
Abstract
During their vegetative growth, plants reiteratively produce leaves, buds, and internodes at the apical end of the shoot. The identity of these organs changes as the shoot develops. Some traits change gradually, but others change in a coordinated fashion, allowing shoot development to be divided into discrete juvenile and adult phases. The transition between these phases is called vegetative phase change. Historically, vegetative phase change has been studied because it is thought to be associated with an increase in reproductive competence. However, this is not true for all species; indeed, heterochronic variation in the timing of vegetative phase change and flowering has made important contributions to plant evolution. In this review, we describe the molecular mechanism of vegetative phase change, how the timing of this process is controlled by endogenous and environmental factors, and its ecological and evolutionary significance.
Keywords: Arabidopsis; SPL genes; developmental timing; flowering time; heterochrony; leaf development; maize; miR156; miR172; shoot development; vegetative phase change.
Copyright © 2023 Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of interests The authors declare no competing interests.
Figures

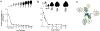
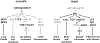

Similar articles
-
Overexpression of lily MicroRNA156-resistant SPL13A stimulates stem elongation and flowering in Lilium formosanum under non-inductive (non-chilling) conditions.Front Plant Sci. 2024 Oct 18;15:1456183. doi: 10.3389/fpls.2024.1456183. eCollection 2024. Front Plant Sci. 2024. PMID: 39494055 Free PMC article.
-
Reproductive competence is regulated independently of vegetative phase change in Arabidopsis thaliana.Curr Biol. 2023 Feb 6;33(3):487-497.e2. doi: 10.1016/j.cub.2022.12.029. Epub 2023 Jan 11. Curr Biol. 2023. PMID: 36634678 Free PMC article.
-
Vegetative phase change and shoot maturation in plants.Curr Top Dev Biol. 2013;105:125-52. doi: 10.1016/B978-0-12-396968-2.00005-1. Curr Top Dev Biol. 2013. PMID: 23962841 Free PMC article. Review.
-
Juvenile Leaves or Adult Leaves: Determinants for Vegetative Phase Change in Flowering Plants.Int J Mol Sci. 2020 Dec 21;21(24):9753. doi: 10.3390/ijms21249753. Int J Mol Sci. 2020. PMID: 33371265 Free PMC article. Review.
-
miRNA control of vegetative phase change in trees.PLoS Genet. 2011 Feb;7(2):e1002012. doi: 10.1371/journal.pgen.1002012. Epub 2011 Feb 24. PLoS Genet. 2011. PMID: 21383862 Free PMC article.
Cited by
-
Overexpression of lily MicroRNA156-resistant SPL13A stimulates stem elongation and flowering in Lilium formosanum under non-inductive (non-chilling) conditions.Front Plant Sci. 2024 Oct 18;15:1456183. doi: 10.3389/fpls.2024.1456183. eCollection 2024. Front Plant Sci. 2024. PMID: 39494055 Free PMC article.
-
Cytokinin depends on GA biosynthesis and signaling to regulate different aspects of vegetative phase change in Arabidopsis.Nat Commun. 2025 Jul 8;16(1):6292. doi: 10.1038/s41467-025-61507-5. Nat Commun. 2025. PMID: 40628766 Free PMC article.
-
SPL13 controls a root apical meristem phase change by triggering oriented cell divisions.Science. 2024 Nov 15;386(6723):eado4298. doi: 10.1126/science.ado4298. Epub 2024 Nov 15. Science. 2024. PMID: 39541454 Free PMC article.
-
Rejuvenation of Mature Ilex paraguariensis Plants Through Serial Rooted Cuttings: Exploring the Roles of miRNAs in Reversing Adult Phase, Promoting Root Formation, and Determining Root Structure.Plants (Basel). 2025 May 30;14(11):1668. doi: 10.3390/plants14111668. Plants (Basel). 2025. PMID: 40508342 Free PMC article.
-
Sorbitol mediates age-dependent changes in apple plant growth strategy through gibberellin signaling.Hortic Res. 2024 Jul 11;11(8):uhae192. doi: 10.1093/hr/uhae192. eCollection 2024 Aug. Hortic Res. 2024. PMID: 39145197 Free PMC article.
References
-
- Allsopp A (1967). Heteroblastic development in vascular plants. Adv. Morphol 6, 127–171. - PubMed
-
- Doorenbos J (1965). Juvenile and adult phases in woody plants. Encyl. Plant Physiol 15, 1222–1235.
-
- Hildebrand F (1875). Ueber die Jungendzustände solcher Pflanzen, welche im Alter vom vegetativen Charakter ihrer Verwandten abweichen. Flora 21, 321–330.
-
- Goebel K (1900). Organography of plants Part I. General organography. (English translation by I. B. Balfour) (Clarendon Press; ).
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

