The role of the motor thalamus in deep brain stimulation for essential tremor
- PMID: 38195310
- PMCID: PMC11103222
- DOI: 10.1016/j.neurot.2023.e00313
The role of the motor thalamus in deep brain stimulation for essential tremor
Abstract
The advent of next-generation technology has significantly advanced the implementation and delivery of Deep Brain Stimulation (DBS) for Essential Tremor (ET), yet controversies persist regarding optimal targets and networks responsible for tremor genesis and suppression. This review consolidates key insights from anatomy, neurology, electrophysiology, and radiology to summarize the current state-of-the-art in DBS for ET. We explore the role of the thalamus in motor function and describe how differences in parcellations and nomenclature have shaped our understanding of the neuroanatomical substrates associated with optimal outcomes. Subsequently, we discuss how seminal studies have propagated the ventral intermediate nucleus (Vim)-centric view of DBS effects and shaped the ongoing debate over thalamic DBS versus stimulation in the posterior subthalamic area (PSA) in ET. We then describe probabilistic- and network-mapping studies instrumental in identifying the local and network substrates subserving tremor control, which suggest that the PSA is the optimal DBS target for tremor suppression in ET. Taken together, DBS offers promising outcomes for ET, with the PSA emerging as a better target for suppression of tremor symptoms. While advanced imaging techniques have substantially improved the identification of anatomical targets within this region, uncertainties persist regarding the distinct anatomical substrates involved in optimal tremor control. Inconsistent subdivisions and nomenclature of motor areas and other subdivisions in the thalamus further obfuscate the interpretation of stimulation results. While loss of benefit and habituation to DBS remain challenging in some patients, refined DBS techniques and closed-loop paradigms may eventually overcome these limitations.
Keywords: Deep brain stimulation; Human thalamic nomenclature; Motor thalamus; Parcellation; Tremor; Vim.
Copyright © 2023 The Authors. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of competing interest The authors declare the following financial interests/personal relationships which may be considered as potential competing interests: Guenther Deuschl reports a relationship with Boston Scientific Corp that includes: consulting or advisory. Guenther Deuschl reports a relationship with Cavion that includes: consulting or advisory. Guenther Deuschl reports a relationship with Functional Neuromodulation that includes: consulting or advisory. Guenther Deuschl reports a relationship with Thieme Medical Publishers that includes: consulting or advisory. Andreas Horn reports a relationship with German Research Foundation that includes: funding grants. Andreas Horn reports a relationship with Deutsches Zentrum für Luft-und Raumfahrt that includes: funding grants.
Figures
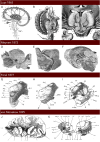

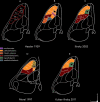
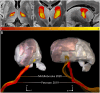
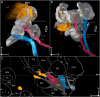

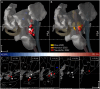
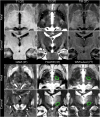
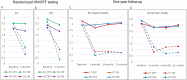
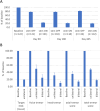
References
-
- Schaltenbrand G., Wahren W., Hassler R.G. Atlas for Stereotaxy of the Human Brain. 2d, rev.Enl. Thieme; Stuttgart: 1977.
-
- Jones E.G., editor. The thalamus [Internet] Springer US; Boston, MA: 1985. http://link.springer.com/10.1007/978-1-4615-1749-8 [cited 2023 Jul 28]. Available from: - DOI
-
- Luys J.B. J.B. Baillière et fils; 1865. Recherches sur le système nerveux cérébro-spinal: sa structure, ses fonctions et ses maladies.
-
- Broca P. Perte de la parole, ramouissement chronique et destruction partielle du lobe antérieur gauche du cerveau | Max Planck Institute. Bulletin de la Société Anthropologique. 1861:235–238.
-
- Nissl F. Die Grosshirnanteile des Kaninchens. Archiv f Psychiatrie. 1913;52:867–953.
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

