Deprivation-Induced Plasticity in the Early Central Circuits of the Rodent Visual, Auditory, and Olfactory Systems
- PMID: 38195533
- PMCID: PMC11059429
- DOI: 10.1523/ENEURO.0435-23.2023
Deprivation-Induced Plasticity in the Early Central Circuits of the Rodent Visual, Auditory, and Olfactory Systems
Abstract
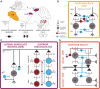
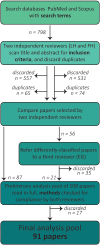
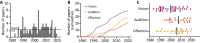
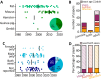
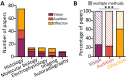
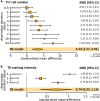
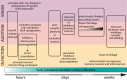
Activity-dependent neuronal plasticity is crucial for animals to adapt to dynamic sensory environments. Traditionally, it has been investigated using deprivation approaches in animal models primarily in sensory cortices. Nevertheless, emerging evidence emphasizes its significance in sensory organs and in subcortical regions where cranial nerves relay information to the brain. Additionally, critical questions started to arise. Do different sensory modalities share common cellular mechanisms for deprivation-induced plasticity at these central entry points? Does the deprivation duration correlate with specific plasticity mechanisms? This study systematically reviews and meta-analyzes research papers that investigated visual, auditory, or olfactory deprivation in rodents of both sexes. It examines the consequences of sensory deprivation in homologous regions at the first central synapse following cranial nerve transmission (vision - lateral geniculate nucleus and superior colliculus; audition - ventral and dorsal cochlear nucleus; olfaction - olfactory bulb). The systematic search yielded 91 papers (39 vision, 22 audition, 30 olfaction), revealing substantial heterogeneity in publication trends, experimental methods, measures of plasticity, and reporting across the sensory modalities. Despite these differences, commonalities emerged when correlating plasticity mechanisms with the duration of sensory deprivation. Short-term deprivation (up to 1 d) reduced activity and increased disinhibition, medium-term deprivation (1 d to a week) involved glial changes and synaptic remodeling, and long-term deprivation (over a week) primarily led to structural alterations. These findings underscore the importance of standardizing methodologies and reporting practices. Additionally, they highlight the value of cross-modal synthesis for understanding how the nervous system, including peripheral, precortical, and cortical areas, respond to and compensate for sensory inputs loss.
Keywords: audition; olfaction; plasticity; rodent; sensory deprivation; vision.
Copyright © 2024 Huang et al.
Figures


Similar articles
-
Human brain plasticity: evidence from sensory deprivation and altered language experience.Prog Brain Res. 2002;138:177-88. doi: 10.1016/S0079-6123(02)38078-6. Prog Brain Res. 2002. PMID: 12432770 Review.
-
Visual Deprivation Selectively Reduces Thalamic Reticular Nucleus-Mediated Inhibition of the Auditory Thalamus in Adults.J Neurosci. 2022 Oct 19;42(42):7921-7930. doi: 10.1523/JNEUROSCI.2032-21.2022. Epub 2022 Sep 7. J Neurosci. 2022. PMID: 36261269 Free PMC article.
-
Competition and convergence between auditory and cross-modal visual inputs to primary auditory cortical areas.J Neurophysiol. 2011 Apr;105(4):1558-73. doi: 10.1152/jn.00407.2010. Epub 2011 Jan 27. J Neurophysiol. 2011. PMID: 21273321 Free PMC article.
-
Brief Sensory Deprivation Triggers Cell Type-Specific Structural and Functional Plasticity in Olfactory Bulb Neurons.J Neurosci. 2021 Mar 10;41(10):2135-2151. doi: 10.1523/JNEUROSCI.1606-20.2020. Epub 2021 Jan 22. J Neurosci. 2021. PMID: 33483429 Free PMC article.
-
Cross-modal plasticity for the spatial processing of sounds in visually deprived subjects.Exp Brain Res. 2009 Jan;192(3):343-58. doi: 10.1007/s00221-008-1553-z. Epub 2008 Sep 2. Exp Brain Res. 2009. PMID: 18762928 Review.
Cited by
-
Olfactory Evoked Potentials and Brain MRI Outcomes in Multiple Sclerosis Patients: A Cross-Sectional Study.J Clin Med. 2024 Dec 29;14(1):141. doi: 10.3390/jcm14010141. J Clin Med. 2024. PMID: 39797224 Free PMC article.
References
-
- Gudden (1870) Experimentaluntersuchungen über das peripherische und centrale Nervensystem. Archiv f Psychiatrie 2:693–723. 10.1007/BF02046772 - DOI
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
